Synaptotagmin-11 facilitates assembly of a presynaptic signaling complex in post-Golgi cargo vesicles
- PMID: 38698221
- PMCID: PMC11169412
- DOI: 10.1038/s44319-024-00147-0
Synaptotagmin-11 facilitates assembly of a presynaptic signaling complex in post-Golgi cargo vesicles
Abstract
GABAB receptors (GBRs), the G protein-coupled receptors for GABA, regulate synaptic transmission throughout the brain. A main synaptic function of GBRs is the gating of Cav2.2-type Ca2+ channels. However, the cellular compartment where stable GBR/Cav2.2 signaling complexes form remains unknown. In this study, we demonstrate that the vesicular protein synaptotagmin-11 (Syt11) binds to both the auxiliary GBR subunit KCTD16 and Cav2.2 channels. Through these dual interactions, Syt11 recruits GBRs and Cav2.2 channels to post-Golgi vesicles, thus facilitating assembly of GBR/Cav2.2 signaling complexes. In addition, Syt11 stabilizes GBRs and Cav2.2 channels at the neuronal plasma membrane by inhibiting constitutive internalization. Neurons of Syt11 knockout mice exhibit deficits in presynaptic GBRs and Cav2.2 channels, reduced neurotransmitter release, and decreased GBR-mediated presynaptic inhibition, highlighting the critical role of Syt11 in the assembly and stable expression of GBR/Cav2.2 complexes. These findings support that Syt11 acts as a vesicular scaffold protein, aiding in the assembly of signaling complexes from low-abundance components within transport vesicles. This mechanism enables insertion of pre-assembled functional signaling units into the synaptic membrane.
Keywords: Cav2.2; GABA-B; KCTD16; Receptor Signaling Complex Assembly; Transport Vesicle.
© 2024. The Author(s).
Conflict of interest statement
BB is a member of the scientific advisory board of Addex Therapeutics, Geneva, a pharmaceutical company focused on the development of allosteric modulators for neurological disorders. All other authors declare no competing interests.
Figures


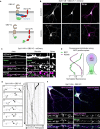

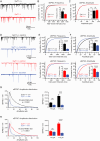
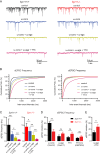

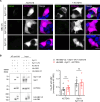


Similar articles
-
α-Neurexins Together with α2δ-1 Auxiliary Subunits Regulate Ca2+ Influx through Cav2.1 Channels.J Neurosci. 2018 Sep 19;38(38):8277-8294. doi: 10.1523/JNEUROSCI.0511-18.2018. Epub 2018 Aug 13. J Neurosci. 2018. PMID: 30104341 Free PMC article.
-
GABAB receptor auxiliary subunits modulate Cav2.3-mediated release from medial habenula terminals.Elife. 2021 Apr 29;10:e68274. doi: 10.7554/eLife.68274. Elife. 2021. PMID: 33913808 Free PMC article.
-
Synaptotagmin-7 Enhances Facilitation of Cav2.1 Calcium Channels.eNeuro. 2022 May 13;9(3):ENEURO.0081-22.2022. doi: 10.1523/ENEURO.0081-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35477860 Free PMC article.
-
Presynaptic Calcium Channels.Int J Mol Sci. 2019 May 6;20(9):2217. doi: 10.3390/ijms20092217. Int J Mol Sci. 2019. PMID: 31064106 Free PMC article. Review.
-
Presynaptic calcium channels.Neurosci Res. 2018 Feb;127:33-44. doi: 10.1016/j.neures.2017.09.012. Epub 2018 Jan 6. Neurosci Res. 2018. PMID: 29317246 Review.
Cited by
-
Genetic implication of GABAB receptors in the etiology of neurological and psychiatric disorders.Front Pharmacol. 2025 Jul 18;16:1634128. doi: 10.3389/fphar.2025.1634128. eCollection 2025. Front Pharmacol. 2025. PMID: 40756990 Free PMC article. Review.
-
Biochemistry and physiology of voltage-gated calcium channel trafficking: a target for gabapentinoid drugs.Open Biol. 2025 Jul;15(7):250013. doi: 10.1098/rsob.250013. Epub 2025 Jul 16. Open Biol. 2025. PMID: 40664238 Free PMC article. Review.
References
-
- Armando S, Quoyer J, Lukashova V, Maiga A, Percherancier Y, Heveker N, Pin JP, Prezeau L, Bouvier M. The chemokine CXC4 and CC2 receptors form homo- and heterooligomers that can engage their signaling G-protein effectors and βarrestin. FASEB J. 2014;28:4509–4523. doi: 10.1096/fj.13-242446. - DOI - PubMed
-
- Bajaj R, Rodriguez BL, Russell WK, Warner AN, Diao L, Wang J, Raso MG, Lu W, Khan K, Solis LS, et al. Impad1 and Syt11 work in an epistatic pathway that regulates EMT-mediated vesicular trafficking to drive lung cancer invasion and metastasis. Cell Rep. 2022;40:111429. doi: 10.1016/j.celrep.2022.111429. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 31003A-152970/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (SNF)
- 310030-205198/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (SNF)
- TRR 152 - project ID 239283807/Deutsche Forschungsgemeinschaft (DFG)
- FA 332/15-1 - project ID 439189341/Deutsche Forschungsgemeinschaft (DFG)
- CIBSS - EXC-2189 - project ID 390939984/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

