F. Nucleatum enhances oral squamous cell carcinoma proliferation via E-cadherin/β-Catenin pathway
- PMID: 38698370
- PMCID: PMC11064238
- DOI: 10.1186/s12903-024-04252-3
F. Nucleatum enhances oral squamous cell carcinoma proliferation via E-cadherin/β-Catenin pathway
Abstract
Background: Fusobacterium nucleatum (F. nucleatum) is a microbial risk factor whose presence increases the risk of oral squamous cell carcinoma (OSCC) progression. However, whether it can promote the proliferation of OSCC cells remains unknown.
Methods: In this study, we investigated F. nucleatum effect on OSCC cell proliferation using in vitro and in vivo experiments.
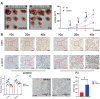
Results: Our results showed that F. nucleatum promoted OSCC cell proliferation, doubling the cell count after 72 h (CCK-8 assay). Cell cycle analysis revealed G2/M phase arrest. F. nucleatum interaction with CDH1 triggered phosphorylation, upregulating downstream protein β-catenin and activating cyclinD1 and Myc. Notably, F. nucleatum did not affect noncancerous cells, unrelated to CDH1 expression levels in CAL27 cells. Overexpression of phosphorylated CDH1 in 293T cells did not upregulate β-catenin and cycle-related genes. In vivo BALB/c nude experiments showed increased tumor volume and Ki-67 proliferation index after F. nucleatum intervention.
Conclusion: Our study suggests that F. nucleatum promotes OSCC cell proliferation through the CDH1/β-catenin pathway, advancing our understanding of its role in OSCC progression and highlighting its potential as a therapeutic target.
Keywords: Fusobacterium nucleatum; CDH1(E-Cadherin); Cell cycle; Cell proliferation; Oral squamous cell carcinoma; Phosphorylation; β-catenin.
© 2024. The Author(s).
Conflict of interest statement
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Figures




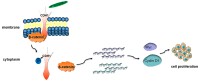
Similar articles
-
Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway.FEBS J. 2020 Sep;287(18):4032-4047. doi: 10.1111/febs.15233. Epub 2020 Feb 12. FEBS J. 2020. PMID: 31997506 Free PMC article.
-
[Fusobacterium nucleatum upregulates ABCG2 by activating the E-cadherin/β-catenin signaling pathway to promote oxaliplatin resistance in colorectal cancer].Zhonghua Zhong Liu Za Zhi. 2025 Apr 23;47(4):329-339. doi: 10.3760/cma.j.cn112152-20240719-00297. Zhonghua Zhong Liu Za Zhi. 2025. PMID: 40268550 Chinese.
-
Fusobacterium nucleatum Caused DNA Damage and Promoted Cell Proliferation by the Ku70/p53 Pathway in Oral Cancer Cells.DNA Cell Biol. 2020 Jan;39(1):144-151. doi: 10.1089/dna.2019.5064. Epub 2019 Nov 25. DNA Cell Biol. 2020. PMID: 31765243 Free PMC article.
-
About a Possible Impact of Endodontic Infections by Fusobacterium nucleatum or Porphyromonas gingivalis on Oral Carcinogenesis: A Literature Overview.Int J Mol Sci. 2024 May 7;25(10):5083. doi: 10.3390/ijms25105083. Int J Mol Sci. 2024. PMID: 38791123 Free PMC article. Review.
-
Fusobacterium nucleatum and oral cancer: a critical review.BMC Cancer. 2021 Nov 13;21(1):1212. doi: 10.1186/s12885-021-08903-4. BMC Cancer. 2021. PMID: 34774023 Free PMC article. Review.
Cited by
-
Splenic volume as a predictor of survival in cancer patients treated with immune checkpoint inhibitors.Front Immunol. 2025 May 30;16:1598484. doi: 10.3389/fimmu.2025.1598484. eCollection 2025. Front Immunol. 2025. PMID: 40519921 Free PMC article.
-
Effects of intratumoral microbiota on tumorigenesis, anti-tumor immunity, and microbe-based cancer therapy.Front Oncol. 2024 Sep 26;14:1429722. doi: 10.3389/fonc.2024.1429722. eCollection 2024. Front Oncol. 2024. PMID: 39391251 Free PMC article. Review.
-
Current Evidence on the Relation Between Microbiota and Oral Cancer-The Role of Fusobacterium nucleatum-A Narrative Review.Cancers (Basel). 2025 Jan 7;17(2):171. doi: 10.3390/cancers17020171. Cancers (Basel). 2025. PMID: 39857953 Free PMC article. Review.
-
Intratumoral Fusobacterium nucleatum is associated with better cancer-specific survival in head and neck cancer patients.J Oral Microbiol. 2025 Apr 1;17(1):2487644. doi: 10.1080/20002297.2025.2487644. eCollection 2025. J Oral Microbiol. 2025. PMID: 40182114 Free PMC article.
-
Dysbiosis of Oral Microbiome: A Key Player in Oral Carcinogenesis? A Critical Review.Biomedicines. 2025 Feb 12;13(2):448. doi: 10.3390/biomedicines13020448. Biomedicines. 2025. PMID: 40002861 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 2022D01C15/Natural Science Foundation of Xinjiang Uygur Autonomous Region
- 2022D01C15/Natural Science Foundation of Xinjiang Uygur Autonomous Region
- 2022D01C15/Natural Science Foundation of Xinjiang Uygur Autonomous Region
- 2022D01C15/Natural Science Foundation of Xinjiang Uygur Autonomous Region
- JYZZ196/Seed Foundation of the Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine
- JYZZ196/Seed Foundation of the Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine
- JYZZ196/Seed Foundation of the Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine
- NCRCO202330/Stomatology Clinical Research Project of Nation Clinical Center forOral Disease
- NCRCO202330/Stomatology Clinical Research Project of Nation Clinical Center forOral Disease
- NCRCO202330/Stomatology Clinical Research Project of Nation Clinical Center forOral Disease
- NCRCO202330/Stomatology Clinical Research Project of Nation Clinical Center forOral Disease
- NCRCO202330/Stomatology Clinical Research Project of Nation Clinical Center forOral Disease
- 81771127/National Natural Science Foundation of China
- 81771127/National Natural Science Foundation of China
- YG2015MS06/Medical Engineering Cross Foundation of Shanghai Jiao Tong University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

