A lava-inspired proteolytic enzyme therapy on cancer with a PEG-based hydrogel enhances tumor distribution and penetration of liposomes
- PMID: 38698399
- PMCID: PMC11067103
- DOI: 10.1186/s12951-024-02468-7
A lava-inspired proteolytic enzyme therapy on cancer with a PEG-based hydrogel enhances tumor distribution and penetration of liposomes
Abstract
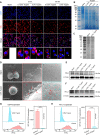
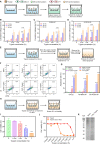
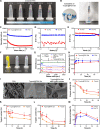
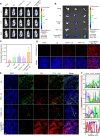
The enhanced permeability and retention (EPR) effect has become the guiding principle for nanomedicine against cancer for a long time. However, several biological barriers severely resist therapeutic agents' penetration and retention into the deep tumor tissues, resulting in poor EPR effect and high tumor mortality. Inspired by lava, we proposed a proteolytic enzyme therapy to improve the tumor distribution and penetration of nanomedicine. A trypsin-crosslinked hydrogel (Trypsin@PSA Gel) was developed to maintain trypsin's activity. The hydrogel postponed trypsin's self-degradation and sustained the release. Trypsin promoted the cellular uptake of nanoformulations in breast cancer cells, enhanced the penetration through endothelial cells, and degraded total and membrane proteins. Proteomic analysis reveals that trypsin affected ECM components and down-regulated multiple pathways associated with cancer progression. Intratumoral injection of Trypsin@PSA Gel significantly increased the distribution of liposomes in tumors and reduced tumor vasculature. Combination treatment with intravenous injection of gambogic acid-loaded liposomes and intratumoral injection of Trypsin@PSA Gel inhibited tumor growth. The current study provides one of the first investigations into the enhanced tumor distribution of liposomes induced by a novel proteolytic enzyme therapy.
Keywords: Enzyme-assisted crosslinking; Hydrogel; Proteolytic enzyme therapy; Tumor distribution of nanomedicine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Zhao M, Mi D, Ferdows BE, Li Y, Wang R, Li J, Patel D, Kong N, Shi S, Tao W. State-of-the-art nanotechnologies for the detection, recovery, analysis and elimination of liquid biopsy components in cancer. Nano Today. 2022;42:101361. doi: 10.1016/j.nantod.2021.101361. - DOI
-
- Dong S, Ma S, Chen H, Tang Z, Song W, Deng M. Nucleobase-crosslinked poly(2-oxazoline) nanoparticles as paclitaxel carriers with enhanced stability and ultra-high drug loading capacity for breast cancer therapy. Asian J Pharm Sci. 2022;17:571–82. doi: 10.1016/j.ajps.2022.04.006. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

