Nirmatrelvir/ritonavir treatment of patients with COVID-19 taking tacrolimus: case series describing the results of drug-drug interactions
- PMID: 38698526
- PMCID: PMC11067642
- DOI: 10.1177/03000605241247705
Nirmatrelvir/ritonavir treatment of patients with COVID-19 taking tacrolimus: case series describing the results of drug-drug interactions
Abstract
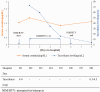
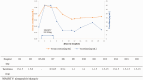
Nirmatrelvir/ritonavir is a novel drug combination that is authorized by the Food and Drug Administration for the treatment of coronavirus disease 2019 (COVID-19). Ritonavir is a cytochrome P450 3A inhibitor and a P-glycoprotein inhibitor that increases the plasma concentration of tacrolimus and other medications. We describe the cases of two patients treated with nirmatrelvir/ritonavir: a patient who had undergone kidney transplantation and another with a history of hematopoietic stem cell transplantation. Toxic concentrations of tacrolimus were induced in both. This case series highlights the risk associated with the concomitant administration of tacrolimus and nirmatrelvir/ritonavir.
Keywords: COVID-19; Nirmatrelvir/ritonavir; drug–drug interaction; hematopoietic stem cell transplantation; kidney transplantation; tacrolimus.
Conflict of interest statement
Declaration of conflicting interestThe authors declare that there is no conflict of interest.
Figures
Similar articles
-
The interaction between nirmatrelvir/ritonavir and sirolimus: a case report of a kidney recipient with renal insufficiency and COVID-19.J Int Med Res. 2025 Jan;53(1):3000605241307845. doi: 10.1177/03000605241307845. J Int Med Res. 2025. PMID: 39749601 Free PMC article.
-
Management and Outcome of COVID-19 Infection Using Nirmatrelvir/Ritonavir in Kidney Transplant Patients.Clin J Am Soc Nephrol. 2023 Jul 1;18(7):913-919. doi: 10.2215/CJN.0000000000000186. Epub 2023 Apr 26. Clin J Am Soc Nephrol. 2023. PMID: 37099447 Free PMC article.
-
Nirmatrelvir/ritonavir treatment in SARS-CoV-2 positive kidney transplant recipients - a case series with four patients.BMC Nephrol. 2023 Apr 15;24(1):99. doi: 10.1186/s12882-023-03154-w. BMC Nephrol. 2023. PMID: 37061677 Free PMC article.
-
Paxlovid (Nirmatrelvir/Ritonavir)-Induced Tacrolimus Toxicity in Organ Transplant Recipients - A Review on Drug Interactions Involving CYP3A Enzymes.Curr Drug Saf. 2025;20(3):291-302. doi: 10.2174/0115748863331165240821194206. Curr Drug Saf. 2025. PMID: 39234905 Review.
-
"Saving lives with nirmatrelvir/ritonavir one transplant patient at a time".Transpl Infect Dis. 2023 Apr;25(2):e14037. doi: 10.1111/tid.14037. Epub 2023 Feb 27. Transpl Infect Dis. 2023. PMID: 36847419 Free PMC article. Review.
Cited by
-
Implementation of an Online Drug-Drug Interaction Screener for the STRIVE Ensitrelvir Trial for COVID-19.Open Forum Infect Dis. 2025 Jun 11;12(7):ofaf327. doi: 10.1093/ofid/ofaf327. eCollection 2025 Jul. Open Forum Infect Dis. 2025. PMID: 40599489 Free PMC article. Clinical Trial.
-
The interaction between nirmatrelvir/ritonavir and sirolimus: a case report of a kidney recipient with renal insufficiency and COVID-19.J Int Med Res. 2025 Jan;53(1):3000605241307845. doi: 10.1177/03000605241307845. J Int Med Res. 2025. PMID: 39749601 Free PMC article.
-
Case report and literature review: management of Paxlovid (nirmatrelvir/ritonavir)-induced acute tacrolimus toxicity in a patient with systemic lupus erythematosus.Front Pharmacol. 2024 Jun 19;15:1364121. doi: 10.3389/fphar.2024.1364121. eCollection 2024. Front Pharmacol. 2024. PMID: 38962309 Free PMC article.
References
-
- Habas K, Nganwuchu C, Shahzad F, et al.. Resolution of coronavirus disease 2019 (COVID-19). Expert Rev Anti Infect Ther 2020; 18: 1201–1211. - PubMed
-
- Pfizer I: Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid. New York: Pfizer, Inc; 2021.
-
- Schutte-Nutgen K, Tholking G, Suwelack B, et al.. Tacrolimus – pharmacokinetic considerations for clinicians. Curr Drug Metab 2018; 19: 342–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical