Ultrasound-Controlled Prodrug Activation: Emerging Strategies in Polymer Mechanochemistry and Sonodynamic Therapy
- PMID: 38698527
- PMCID: PMC11653258
- DOI: 10.1021/acsabm.4c00150
Ultrasound-Controlled Prodrug Activation: Emerging Strategies in Polymer Mechanochemistry and Sonodynamic Therapy
Abstract
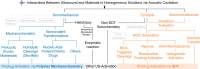
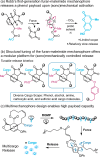
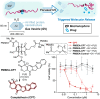
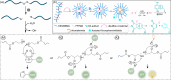
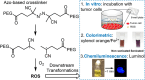
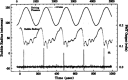
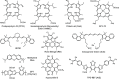
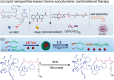
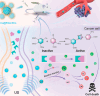
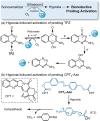
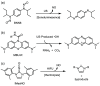
Ultrasound has gained prominence in biomedical applications due to its noninvasive nature and ability to penetrate deep tissue with spatial and temporal resolution. The burgeoning field of ultrasound-responsive prodrug systems exploits the mechanical and chemical effects of ultrasonication for the controlled activation of prodrugs. In polymer mechanochemistry, materials scientists exploit the sonomechanical effect of acoustic cavitation to mechanochemically activate force-sensitive prodrugs. On the other hand, researchers in the field of sonodynamic therapy adopt fundamentally distinct methodologies, utilizing the sonochemical effect (e.g., generation of reactive oxygen species) of ultrasound in the presence of sonosensitizers to induce chemical transformations that activate prodrugs. This cross-disciplinary review comprehensively examines these two divergent yet interrelated approaches, both of which originated from acoustic cavitation. It highlights molecular and materials design strategies and potential applications in diverse therapeutic contexts, from chemotherapy to immunotherapy and gene therapy methods, and discusses future directions in this rapidly advancing domain.
Keywords: drug delivery; mechanophores; polymer mechanochemistry; prodrugs; sonochemistry; sonodynamic therapy; sonosensitizers; ultrasound.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Ultrasound controlled mechanophore activation in hydrogels for cancer therapy.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2109791119. doi: 10.1073/pnas.2109791119. Proc Natl Acad Sci U S A. 2022. PMID: 35046028 Free PMC article.
-
A novel self-targeting theranostic nanoplatform for photoacoustic imaging-monitored and enhanced chemo-sonodynamic therapy.J Mater Chem B. 2021 Jul 14;9(27):5547-5559. doi: 10.1039/d1tb01025e. J Mater Chem B. 2021. PMID: 34165487
-
Self-Indicating Polymer Prodrug Nanoparticles for pH-Responsive Drug Delivery in Cancer Cells and Real-Time Monitoring of Drug Release.ACS Appl Bio Mater. 2024 Sep 16;7(9):5810-5822. doi: 10.1021/acsabm.4c00878. Epub 2024 Aug 26. ACS Appl Bio Mater. 2024. PMID: 39186444 Review.
-
A glutathione-activated bismuth-gallic acid metal-organic framework nano-prodrug for enhanced sonodynamic therapy of breast tumor.J Colloid Interface Sci. 2025 Feb;679(Pt A):214-223. doi: 10.1016/j.jcis.2024.09.233. Epub 2024 Sep 29. J Colloid Interface Sci. 2025. PMID: 39362146
-
Sonodynamic and Acoustically Responsive Nanodrug Delivery System: Cancer Application.Int J Nanomedicine. 2024 Nov 11;19:11767-11788. doi: 10.2147/IJN.S496028. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39553460 Free PMC article. Review.
Cited by
-
Dual-Gating Strategy: Ultrasound Activation of TRPV2 Channels and Borate-Glass-Induced Calcium Overload for Tumor Suppression.Adv Sci (Weinh). 2025 Apr;12(16):e2414676. doi: 10.1002/advs.202414676. Epub 2025 Feb 27. Adv Sci (Weinh). 2025. PMID: 40013983 Free PMC article.
-
Subcellular Cavitation Bubbles Induce Cellular Mechanolysis and Collective Wound Healing in Ultrasound-Inflicted Cell Ablation.Adv Sci (Weinh). 2025 Mar;12(11):e2410760. doi: 10.1002/advs.202410760. Epub 2025 Jan 30. Adv Sci (Weinh). 2025. PMID: 39887946 Free PMC article.
-
In-situ monitoring of polymer mechanochemistry: what can be learned from small molecule systems.Front Chem. 2024 Oct 16;12:1490847. doi: 10.3389/fchem.2024.1490847. eCollection 2024. Front Chem. 2024. PMID: 39478993 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
