The changing of α5-GABAA receptors expression and distribution participate in sevoflurane-induced learning and memory impairment in young mice
- PMID: 38698533
- PMCID: PMC11066188
- DOI: 10.1111/cns.14716
The changing of α5-GABAA receptors expression and distribution participate in sevoflurane-induced learning and memory impairment in young mice
Abstract
Background: Sevoflurane is a superior agent for maintaining anesthesia during surgical procedures. However, the neurotoxic mechanisms of clinical concentration remain poorly understood. Sevoflurane can interfere with the normal function of neurons and synapses and impair cognitive function by acting on α5-GABAAR.
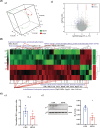
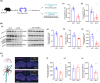
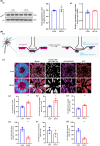
Methods: Using MWM test, we evaluated cognitive abilities in mice following 1 h of anesthesia with 2.7%-3% sevoflurane. Based on hippocampal transcriptome analysis, we analyzed the differential genes and IL-6 24 h post-anesthesia. Western blot and RT-PCR were performed to measure the levels of α5-GABAAR, Radixin, P-ERM, P-Radixin, Gephyrin, IL-6, and ROCK. The spatial distribution and expression of α5-GABAAR on neuronal somata were analyzed using histological and three-dimensional imaging techniques.
Results: MWM test indicated that partial long-term learning and memory impairment. Combining molecular biology and histological analysis, our studies have demonstrated that sevoflurane induces immunosuppression, characterized by reduced IL-6 expression levels, and that enhanced Radixin dephosphorylation undermines the microstructural stability of α5-GABAAR, leading to its dissociation from synaptic exterior and resulting in a disordered distribution in α5-GABAAR expression within neuronal cell bodies. On the synaptic cleft, the expression level of α5-GABAAR remained unchanged, the spatial distribution became more compact, with an increased fluorescence intensity per voxel. On the extra-synaptic space, the expression level of α5-GABAAR decreased within unchanged spatial distribution, accompanied by an increased fluorescence intensity per voxel.
Conclusion: Dysregulated α5-GABAAR expression and distribution contributes to sevoflurane-induced partial long-term learning and memory impairment, which lays the foundation for elucidating the underlying mechanisms in future studies.
Keywords: changing; expression and distribution; neurotoxic mechanism; partial long‐term learning and memory impairment; sevoflurane; α5‐GABAAR.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The author(s) declare that they have no potential conflicts of interest.
Figures


Similar articles
-
Sevoflurane-induced overexpression of extrasynaptic α5-GABAAR via the RhoA/ROCK2 pathway impairs cognitive function in aged mice.Aging Cell. 2024 Sep;23(9):e14209. doi: 10.1111/acel.14209. Epub 2024 Jun 2. Aging Cell. 2024. PMID: 38825816 Free PMC article.
-
Restoration of Extrasynaptic/Synaptic GABAAR-α5 Localization Improves Sevoflurane-Induced Early Memory Impairment in Aged Mice.Neurosci Bull. 2025 Jun 21. doi: 10.1007/s12264-025-01436-x. Online ahead of print. Neurosci Bull. 2025. PMID: 40542952
-
Input-Specific Synaptic Location and Function of the α5 GABAA Receptor Subunit in the Mouse CA1 Hippocampal Neurons.J Neurosci. 2019 Jan 30;39(5):788-801. doi: 10.1523/JNEUROSCI.0567-18.2018. Epub 2018 Dec 6. J Neurosci. 2019. PMID: 30523065 Free PMC article.
-
Molecular Mechanism of Sevoflurane Affecting Fetal Nervous System's Development Through the GABAAR/Sirt 1 Pathway.Altern Ther Health Med. 2024 May;30(5):136-140. Altern Ther Health Med. 2024. PMID: 38743887
-
Diversity matters: combinatorial information coding by GABAA receptor subunits during spatial learning and its allosteric modulation.Cell Signal. 2018 Oct;50:142-159. doi: 10.1016/j.cellsig.2018.07.003. Epub 2018 Jul 11. Cell Signal. 2018. PMID: 30006122 Review.
Cited by
-
Radixin: Roles in the Nervous System and Beyond.Biomedicines. 2024 Oct 15;12(10):2341. doi: 10.3390/biomedicines12102341. Biomedicines. 2024. PMID: 39457653 Free PMC article. Review.
-
Dual effects of GABA A R agonist anesthetics in neurodevelopment and vulnerable brains: From neurotoxic to therapeutic effects.Neural Regen Res. 2026 Jan 1;21(1):81-95. doi: 10.4103/NRR.NRR-D-24-00828. Epub 2024 Dec 7. Neural Regen Res. 2026. PMID: 39665822 Free PMC article.
-
ICU patient-on-a-chip emulating orchestration of mast cells and cerebral organoids in neuroinflammation.Commun Biol. 2024 Dec 5;7(1):1627. doi: 10.1038/s42003-024-07313-z. Commun Biol. 2024. PMID: 39639082 Free PMC article.
References
-
- Aoki N, Suwa T, Kawashima H, et al. Sevoflurane in electroconvulsive therapy: a systematic review and meta‐analysis of randomised trials. J Psychiatr Res. 2021;141:16‐25. - PubMed
-
- Apai C, Shah R, Tran K, Pandya SS. Anesthesia and the developing brain: a review of sevoflurane‐induced neurotoxicity in pediatric populations. Clin Ther. 2021;43(4):762‐778. - PubMed
-
- Wang TT, Lu HF, Poon YY, et al. Sevoflurane versus desflurane for early postoperative vomiting after general anesthesia in hospitalized adults: a systematic review and meta‐analysis of randomized controlled trials. J Clin Anesth. 2021;75:110464. - PubMed
-
- Geng YJ, Wu QH, Zhang RQ. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: a randomized controlled trial. J Clin Anesth. 2017;38:165‐171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

