Erasable and Field Programmable DNA Circuits Based on Configurable Logic Blocks
- PMID: 38698560
- PMCID: PMC11234411
- DOI: 10.1002/advs.202400011
Erasable and Field Programmable DNA Circuits Based on Configurable Logic Blocks
Abstract
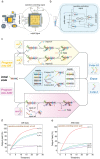
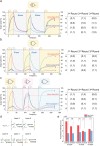
DNA is commonly employed as a substrate for the building of artificial logic networks due to its excellent biocompatibility and programmability. Till now, DNA logic circuits are rapidly evolving to accomplish advanced operations. Nonetheless, nowadays, most DNA circuits remain to be disposable and lack of field programmability and thereby limits their practicability. Herein, inspired by the Configurable Logic Block (CLB), the CLB-based erasable field-programmable DNA circuit that uses clip strands as its operation-controlling signals is presented. It enables users to realize diverse functions with limited hardware. CLB-based basic logic gates (OR and AND) are first constructed and demonstrated their erasability and field programmability. Furthermore, by adding the appropriate operation-controlling strands, multiple rounds of programming are achieved among five different logic operations on a two-layer circuit. Subsequently, a circuit is successfully built to implement two fundamental binary calculators: half-adder and half-subtractor, proving that the design can imitate silicon-based binary circuits. Finally, a comprehensive CLB-based circuit is built that enables multiple rounds of switch among seven different logic operations including half-adding and half-subtracting. Overall, the CLB-based erasable field-programmable circuit immensely enhances their practicability. It is believed that design can be widely used in DNA logic networks due to its efficiency and convenience.
Keywords: DNA logic circuits; DNA nanotechnology; clip‐mediated strand displacement reaction; configurable logic block; field programming.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Scaling Up Multi-bit DNA Full Adder Circuits with Minimal Strand Displacement Reactions.J Am Chem Soc. 2022 Jun 1;144(21):9479-9488. doi: 10.1021/jacs.2c03258. Epub 2022 May 22. J Am Chem Soc. 2022. PMID: 35603742
-
Programmable DNA Nanoindicator-Based Platform for Large-Scale Square Root Logic Biocomputing.Small. 2019 Dec;15(49):e1903489. doi: 10.1002/smll.201903489. Epub 2019 Oct 29. Small. 2019. PMID: 31661189
-
Programmable nanowire circuits for nanoprocessors.Nature. 2011 Feb 10;470(7333):240-4. doi: 10.1038/nature09749. Nature. 2011. PMID: 21307937
-
DNA Logic Circuits for Cancer Theranostics.Small. 2022 May;18(20):e2108008. doi: 10.1002/smll.202108008. Epub 2022 Mar 7. Small. 2022. PMID: 35254723 Review.
-
Exploring DNA Computers: Advances in Storage, Cryptography and Logic Circuits.Chembiochem. 2025 Jan 2;26(1):e202400670. doi: 10.1002/cbic.202400670. Epub 2024 Nov 6. Chembiochem. 2025. PMID: 39365708 Review.
References
-
- Song X., Reif J., ACS Nano 2019, 13, 6256. - PubMed
-
- Zhao S., Yu L., Yang S., Tang X., Chang K., Chen M., Nanoscale Horiz. 2021, 6, 298. - PubMed
-
- Qian L., Winfree E., Bruck J., Nature 2011, 475, 368. - PubMed
-
- Zhang C., Ge L., Zhuang Y., Shen Z., Zhong Z., Zhang Z., You X., Sci. China Inf. Sci. 2019, 62, 61301.
MeSH terms
Substances
Grants and funding
- 2023YFE0210200,2021YFC2701402/National Key Research and Development Program of China
- HPKL2023019,HPKL2023036/the open research fund of the Hunan Provincial Key Laboratory of Regional Hereditary Birth Defects Prevention and Control
- MHP/240/23/the Hong Kong Innovation and Technology Fund, the Mainland-Hong Kong Joint Funding Scheme (Platform)
- HZQB-KCZYZ-2021017/the Hetao Shenzhen-Hong Kong Science and Technology Innovation Cooperation Zone Shenzhen Park Project
- 2018YFA0901104/the National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
