Clinical outcomes of pomalidomide-based and daratumumab-based therapies in patients with relapsed/refractory multiple myeloma: A real-world cohort study in China
- PMID: 38698679
- PMCID: PMC11066492
- DOI: 10.1002/cam4.7232
Clinical outcomes of pomalidomide-based and daratumumab-based therapies in patients with relapsed/refractory multiple myeloma: A real-world cohort study in China
Abstract
Background: Comparative investigations evaluating the efficacy of pomalidomide-based (Pom-based) versus daratumumab-based (Dara-based) therapies in patients with relapsed/refractory multiple myeloma (RRMM) remain scarce, both in randomized controlled trials and real-world studies.
Methods: This retrospective cohort study included 140 RRMM patients treated with Pom-based or Dara-based or a combination of pomalidomide and daratumumab (DPd) regimens in a Chinese tertiary hospital between December 2018 and July 2023.
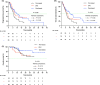
Results: The overall response rates (ORR) for Pom-based (n = 48), Dara-based (n = 68), and DPd (n = 24) groups were 57.8%, 84.6%, and 75.0%, respectively (p = 0.007). At data cutoff on August 1, 2023, the median progression-free survival (PFS) was 5.7 months (95% CI: 5.0-6.5) for the Pom-based group, 10.5 months (5.2-15.8) for the Dara-based group, and 6.7 months (4.0-9.3) for the DPd group (p = 0.056). Multivariate analysis identified treatment regimens (Dara-based vs. Pom-based, DPd vs. Pom-based) and Eastern Cooperative Oncology Group performance status (ECOG PS) as independent prognostic factors for PFS. In the subgroups of patients aged >65 years, with ECOG PS ≥2, lines of therapy ≥2, extramedullary disease or double-refractory disease (refractory to both lenalidomide and proteasome inhibitors), the superiority of Dara-based regimens over Pom-based regimens was not evident. A higher incidence of infections was observed in patients receiving Dara-based and DPd regimens (Pom-based 39.6% vs. Dara-based 64.7% vs. DPd 70.8%, p = 0.009).
Conclusions: In real-world settings, Pom-based, Dara-based, and DPd therapies exhibited favorable efficacy in patients with RRMM. Dara-based therapy yielded superior clinical response and PFS compared to Pom-based therapy.
Keywords: daratumumab; multiple myeloma; pomalidomide; recurrence; refractory.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no relevant financial or non‐financial interests to disclose.
Figures


References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046‐1060. - PubMed
-
- Nooka AK, Kastritis E, Dimopoulos MA, Lonial S. Treatment options for relapsed and refractory multiple myeloma. Blood. 2015;125(20):3085‐3099. - PubMed
-
- Blair HA. Daratumumab: a review in relapsed and/or refractory multiple myeloma. Drugs. 2017;77(18):2013‐2024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

