A PD-1highCD4+ T Cell Population With a Cytotoxic Phenotype is Associated With Interstitial Lung Disease in Systemic Sclerosis
- PMID: 38698736
- PMCID: PMC11246828
- DOI: 10.1002/acr2.11671
A PD-1highCD4+ T Cell Population With a Cytotoxic Phenotype is Associated With Interstitial Lung Disease in Systemic Sclerosis
Abstract
Objective: T cells contribute to tissue injury in systemic sclerosis (SSc), yet the specific T cell subsets expanded in patients with SSc remain incompletely defined. Here we evaluated specific phenotypes and functions of peripheral helper T (Tph) and follicular helper T (Tfh) cells, which have been implicated in autoantibody production, and assessed their associations with clinical features in a well-characterized cohort of patients with SSc.
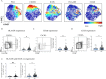
Methods: Mass cytometry of T cells from peripheral blood mononuclear cells of patients with SSc and controls were evaluated using t-distributed stochastic neighbor embedding visualization, biaxial gating, and marker expression levels. Findings were validated with flow cytometry and in vitro assays.
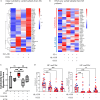
Results: The frequencies of PD-1highCXCR5+ Tfh cells and PD-1highCXCR5- Tph cells were similar in patients with SSc and controls. t-distributed stochastic neighbor embedding visualization (tSNE) revealed distinct populations within the PD-1highCXCR5- cells distinguished by expression of HLA-DR and inducible costimulator (ICOS). Among PD-1highCXCR5- cells, only the HLA-DR+ICOS- cell population was expanded in patients with SSc. Cytometric and RNA sequencing analyses indicated that these cells expressed cytotoxic rather than B cell helper features. HLA-DR+ICOS- PD-1highCXCR5- cells were less potent in inducing B cell plasmablast differentiation and antibody production than comparator T helper cell populations. HLA-DR+ICOS-PD-1highCXCR5- cells were significantly associated with the presence and severity of interstitial lung disease among patients with SSc.
Conclusion: Among PD-1highCXCR5- T cells, a subset of HLA-DR+ICOS- cells with cytotoxic features is specifically expanded in patients with SSc and is significantly associated with interstitial lung disease severity. This potential cytotoxicity appearing in the CD4 T cell population can be evaluated as a prognostic disease biomarker in patients with SSc.
© 2024 The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures



References
-
- Perelas A, Silver RM, Arrossi AV, et al. Systemic sclerosis‐associated interstitial lung disease. Lancet Respir Med 2020;8(3):304–320. - PubMed
-
- O'Reilly S, Hügle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford) 2012;51(9):1540–1549. - PubMed
-
- Frieri M, Angadi C, Paolano A, et al. Altered T cell subpopulations and lymphocytes expressing natural killer cell phenotypes in patients with progressive systemic sclerosis. J Allergy Clin Immunol 1991;87(4):773–779. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

