Comparison of SP263 and 22C3 pharmDx assays to test programmed death ligand-1 (PD-L1) expression in surgically resected non-small cell lung cancer
- PMID: 38698758
- PMCID: PMC11168908
- DOI: 10.1111/1759-7714.15319
Comparison of SP263 and 22C3 pharmDx assays to test programmed death ligand-1 (PD-L1) expression in surgically resected non-small cell lung cancer
Abstract
Background: Atezolizumab, one of the immune checkpoint inhibitors, has been approved as an adjuvant treatment following resection and platinum-based chemotherapy in patients with stage II-IIIA non-small cell lung cancer with 1% or more programmed death ligand-1 (PD-L1) expression. The Food and Drug Administration (FDA) has approved SP263 as a companion diagnostic assay for adjuvant treatment with atezolizumab; however, in clinical practice, the 22C3 assay is most commonly used for advanced non-small cell lung cancer. Therefore, our study aimed to compare two PD-L1 assays, SP263 and 22C3, to evaluate whether 22C3 could replace SP263 when deciding whether to administer adjuvant atezolizumab.
Methods: We retrospectively and prospectively analyzed 98 patients who underwent surgical resection at Kanagawa Cancer Center (Japan). An immunohistochemistry assay was performed for all the cases with both SP263 and 22C3. We statistically analyzed the concordance of PD-L1 expression between SP263 and 22C3 assays.
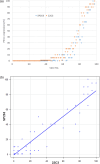
Results: The concordance between the two assays using Cohen's kappa was κ = 0.670 (95% CI: 0.522-0.818) at the 1% cutoff and κ = 0.796 (95% CI: 0.639-0.954) at the 50% cutoff. The Spearman correlation coefficient of 0.874 (p < 0.01) indicated high concordance. PD-L1 expression with 22C3 resulted slightly higher than that with SP263.
Conclusions: This study showed a high concordance of PD-L1 expression with the SP263 and 22C3 assays. Further studies examining the therapeutic effects of adjuvant atezolizumab are required.
Keywords: PD‐L1 immunohistochemistry assay; immune checkpoint inhibitors (ICIs); non‐small cell lung cancer (NSCLC); programmed death ligand‐1.
© 2024 The Authors. Thoracic Cancer published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Shuji Murakami reports personal fees from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Taiho Pharmaceutical, Ono Pharmaceutical, and Riken Genesis. Terufumi Kato reports grants and personal fees from MSD, Novartis, Ono Pharmaceutical, Pfizer, and Taiho Pharmaceutical, personal fees from Daiichi Sankyo, F. Hoffmann‐La Roche, Nippon Kayaku, Nitto Denko, Shionogi Pharmaceutical, Sumitomo Dainippon, and Takeda, and grants from Astellas, Kyorin, Kyowa Kirin, and Regeneron. Haruhiro Saito reports grants from Chugai Pharmaceutical and AstraZeneca, and personal fees from Ono Pharmaceutical, Nippon Boehringer Ingelheim, MSD, and Novartis Pharma.
Figures
Similar articles
-
Evaluation of the diagnostic concordance of FDA-approved PD-L1 assays in clear cell renal cell carcinoma.Sci Rep. 2025 Jul 1;15(1):21253. doi: 10.1038/s41598-025-05697-4. Sci Rep. 2025. PMID: 40593071 Free PMC article.
-
PD-L1 expression and its association with clinicopathological and computed tomography features in surgically resected non-small cell lung cancer: a retrospective cohort study.Sci Rep. 2025 Jul 7;15(1):24323. doi: 10.1038/s41598-025-10437-9. Sci Rep. 2025. PMID: 40624192 Free PMC article.
-
Clinical significance of inter-assay discrepancy in PD-L1 evaluation for the efficacy of pembrolizumab in advanced NSCLC with high PD-L1 expression.Lung Cancer. 2024 May;191:107788. doi: 10.1016/j.lungcan.2024.107788. Epub 2024 Apr 6. Lung Cancer. 2024. PMID: 38593478
-
Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis.Oncologist. 2017 Apr;22(4):470-479. doi: 10.1634/theoncologist.2016-0419. Epub 2017 Mar 8. Oncologist. 2017. PMID: 28275115 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
Cited by
-
Application of the PDCA cycle in companion diagnostic PD-L1 SP263 immunohistochemical testing.BMC Cancer. 2025 Jul 12;25(1):1167. doi: 10.1186/s12885-025-14572-4. BMC Cancer. 2025. PMID: 40652196 Free PMC article.
-
Comparison of PD-L1 Expression Between Preoperative Biopsy Specimens and Surgical Specimens in Non-Small Cell Lung Cancer.Cancers (Basel). 2025 Jan 25;17(3):398. doi: 10.3390/cancers17030398. Cancers (Basel). 2025. PMID: 39941767 Free PMC article.
References
-
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. - PubMed
-
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. - PubMed
-
- Herbst RS, Baas P, Kim DW, Felip E, Perez‐Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials