MLXIPL associated with tumor-infiltrating CD8+ T cells is involved in poor prostate cancer prognosis
- PMID: 38698844
- PMCID: PMC11063283
- DOI: 10.3389/fimmu.2024.1364329
MLXIPL associated with tumor-infiltrating CD8+ T cells is involved in poor prostate cancer prognosis
Abstract
Introduction: Within tumor microenvironment, the presence of preexisting antitumor CD8+ T Q7 cells have been shown to be associated with a favorable prognosis in most solid cancers. However, in the case of prostate cancer (PCa), they have been linked to a negative impact on prognosis.
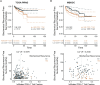
Methods: To gain a deeper understanding of the contribution of infiltrating CD8+ T cells to poor prognosis in PCa, the infiltration levelsof CD8+ T cells were estimated using the TCGA PRAD (The Cancer Genome Atlas Prostate Adenocarcinoma dataset) and MSKCC (Memorial Sloan Kettering Cancer Center) cohorts.
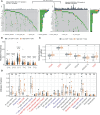
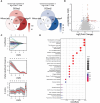
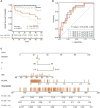
Results: Bioinformatic analyses revealed that CD8+ T cells likely influence PCa prognosis through increased expression of immune checkpoint molecules and enhanced recruitment of regulatory T cells. The MLXIPL was identified as the gene expressed in response to CD8+ T cell infiltration and was found to be associated with PCa prognosis. The prognostic role of MLXIPL was examined in two cohorts: TCGA PRAD (p = 2.3E-02) and the MSKCC cohort (p = 1.6E-02). Subsequently, MLXIPL was confirmed to be associated with an unfavorable prognosis in PCa, as evidenced by an independent cohort study (hazard ratio [HR] = 2.57, 95% CI: 1.42- 4.65, p = 1.76E-03).
Discussion: In summary, the findings suggested that MLXIPL related to tumor-infiltrating CD8+ T cells facilitated a poor prognosis in PCa.
Keywords: CD8+ T cell; MLXIPL; cohort study; prognosis; prostate cancer.
Copyright © 2024 Fan, Ge, Niu, Li, Qi, Zhu and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
WHSC1/NSD2 regulates immune infiltration in prostate cancer.J Immunother Cancer. 2021 Feb;9(2):e001374. doi: 10.1136/jitc-2020-001374. J Immunother Cancer. 2021. PMID: 33589522 Free PMC article.
-
Network models of prostate cancer immune microenvironments identify ROMO1 as heterogeneity and prognostic marker.Sci Rep. 2022 Jan 7;12(1):192. doi: 10.1038/s41598-021-03946-w. Sci Rep. 2022. PMID: 34996995 Free PMC article.
-
The signature of cuproptosis-related immune genes predicts the tumor microenvironment and prognosis of prostate adenocarcinoma.Front Immunol. 2023 Aug 2;14:1181370. doi: 10.3389/fimmu.2023.1181370. eCollection 2023. Front Immunol. 2023. PMID: 37600770 Free PMC article.
-
Effect of tumor CD276 expression on infiltrating immune cells and clinicopathological features of prostate cancer.Prostate Cancer Prostatic Dis. 2024 Dec;27(4):783-785. doi: 10.1038/s41391-023-00690-2. Epub 2023 Jun 28. Prostate Cancer Prostatic Dis. 2024. PMID: 37380802
-
A Systematic Review of the Tumor-Infiltrating CD8+ T-Cells/PD-L1 Axis in High-Grade Glial Tumors: Toward Personalized Immuno-Oncology.Front Immunol. 2021 Sep 17;12:734956. doi: 10.3389/fimmu.2021.734956. eCollection 2021. Front Immunol. 2021. PMID: 34603316 Free PMC article.
Cited by
-
Bolstering CD8+ T Cells' Antitumor Immunity: A Promising Strategy to Improve the Response to Advanced Prostate Cancer Treatment.Biology (Basel). 2025 May 14;14(5):544. doi: 10.3390/biology14050544. Biology (Basel). 2025. PMID: 40427733 Free PMC article. Review.
-
A Whole-Transcriptomic Analysis of Canine Oral Melanoma: A Chance to Disclose the Radiotherapy Effect and Outcome-Associated Gene Signature.Genes (Basel). 2024 Aug 13;15(8):1065. doi: 10.3390/genes15081065. Genes (Basel). 2024. PMID: 39202425 Free PMC article.
-
Exploring the interaction between immune cells in the prostate cancer microenvironment combining weighted correlation gene network analysis and single-cell sequencing: An integrated bioinformatics analysis.Discov Oncol. 2024 Sep 30;15(1):513. doi: 10.1007/s12672-024-01399-x. Discov Oncol. 2024. PMID: 39349877 Free PMC article.
References
-
- Miszczyk M, Rajwa P, Yanagisawa T, Nowicka Z, Shim SR, Laukhtina E, et al. . The efficacy and safety of metastasis-directed therapy in patients with prostate cancer: A systematic review and meta-analysis of prospective studies. Eur Urol. (2024) 85:125–38. doi: 10.1016/j.eururo.2023.10.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

