Immunogenicity of COVID-19 booster vaccination in IEI patients and their one year clinical follow-up after start of the COVID-19 vaccination program
- PMID: 38698851
- PMCID: PMC11063285
- DOI: 10.3389/fimmu.2024.1390022
Immunogenicity of COVID-19 booster vaccination in IEI patients and their one year clinical follow-up after start of the COVID-19 vaccination program
Abstract
Purpose: Previous studies have demonstrated that the majority of patients with an inborn error of immunity (IEI) develop a spike (S)-specific IgG antibody and T-cell response after two doses of the mRNA-1273 COVID-19 vaccine, but little is known about the response to a booster vaccination. We studied the immune responses 8 weeks after booster vaccination with mRNA-based COVID-19 vaccines in 171 IEI patients. Moreover, we evaluated the clinical outcomes in these patients one year after the start of the Dutch COVID-19 vaccination campaign.
Methods: This study was embedded in a large prospective multicenter study investigating the immunogenicity of COVID-19 mRNA-based vaccines in IEI (VACOPID study). Blood samples were taken from 244 participants 8 weeks after booster vaccination. These participants included 171 IEI patients (X-linked agammaglobulinemia (XLA;N=11), combined immunodeficiency (CID;N=4), common variable immunodeficiency (CVID;N=45), isolated or undefined antibody deficiencies (N=108) and phagocyte defects (N=3)) and 73 controls. SARS-CoV-2-specific IgG titers, neutralizing antibodies, and T-cell responses were evaluated. One year after the start of the COVID-19 vaccination program, 334 study participants (239 IEI patients and 95 controls) completed a questionnaire to supplement their clinical data focusing on SARS-CoV-2 infections.
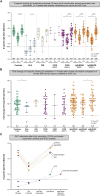
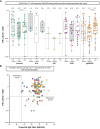
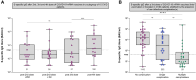
Results: After booster vaccination, S-specific IgG titers increased in all COVID-19 naive IEI cohorts and controls, when compared to titers at 6 months after the priming regimen. The fold-increases did not differ between controls and IEI cohorts. SARS-CoV-2-specific T-cell responses also increased equally in all cohorts after booster vaccination compared to 6 months after the priming regimen. Most SARS-CoV-2 infections during the study period occurred in the period when the Omicron variant had become dominant. The clinical course of these infections was mild, although IEI patients experienced more frequent fever and dyspnea compared to controls and their symptoms persisted longer.
Conclusion: Our study demonstrates that mRNA-based booster vaccination induces robust recall of memory B-cell and T-cell responses in most IEI patients. One-year clinical follow-up demonstrated that SARS-CoV-2 infections in IEI patients were mild. Given our results, we support booster campaigns with newer variant-specific COVID-19 booster vaccines to IEI patients with milder phenotypes.
Keywords: SARS-CoV-2; T-cell response; antibody response; booster vaccination; immunogenicity; inborn errors of immunity; mRNA-1273 COVID-19 vaccine; primary immunodeficiency disorders.
Copyright © 2024 van Leeuwen, Grobben, GeurtsvanKessel, Ellerbroek, de Bree, Potjewijd, Rutgers, Jolink, van de Veerdonk, van Gils, de Vries, Dalm and VACOPID Research Group.
Conflict of interest statement
JP received a grant from GlaxoSmithKline for an improvement of clinical care project and received support from Prothva Biosolutions for attending meetings and cover of travel expenses. JP participates in an Advisory Board for Janssen. FV received a grant from ZonMW for a study on lanadelumab in COVID-19, and consulting fees from GSK made to his department. VD received consulting fees from GlaxoSmithKline, Pharming NV for Advisory board meetings and honoraria for lectures from Takeda Pharmaceutical Company, Kedrion, AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

