Aberrant CD8+T cells drive reproductive dysfunction in female mice with elevated IFN-γ levels
- PMID: 38698852
- PMCID: PMC11064017
- DOI: 10.3389/fimmu.2024.1368572
Aberrant CD8+T cells drive reproductive dysfunction in female mice with elevated IFN-γ levels
Abstract
Introduction: Interferon-gamma (IFN-γ) is pivotal in orchestrating immune responses during healthy pregnancy. However, its dysregulation, often due to autoimmunity, infections, or chronic inflammatory conditions, is implicated in adverse reproductive outcomes such as pregnancy failure or infertility. Additionally, the underlying immunological mechanisms remain elusive.
Methods: Here, we explore the impact of systemic IFN-γ elevation on cytotoxic T cell responses in female reproduction utilizing a systemic lupus-prone mouse model with impaired IFN-γ degradation.
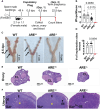
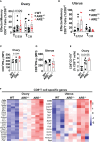
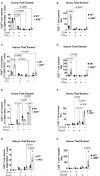
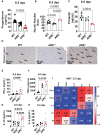
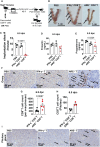
Results: Our findings reveal that heightened IFN-γ levels triggered the infiltration of CD8+T cells in the pituitary gland and female reproductive tract (FRT), resulting in prolactin deficiency and subsequent infertility. Furthermore, we demonstrate that chronic IFN-γ elevation increases effector memory CD8+T cells in the murine ovary and uterus.
Discussion: These insights broaden our understanding of the role of elevated IFN-γ in female reproductive dysfunction and suggest CD8+T cells as potential immunotherapeutic targets in female reproductive disorders associated with chronic systemic IFN-γ elevation.
Keywords: CD8+ T cells; hypophysitis; implantation failure; interferon-gamma (IFN-γ); luteinization defect; pregnancy; prolactin deficiency; tissue-resident memory.
Copyright © 2024 Bafor, Erwin-Cohen, Martin, Baker, Kimmel, Duverger, Fenimore, Ramba, Spindel, Hess, Sanford, Lazarevic, Benayoun, Young and Valencia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

