Changes in the N-glycosylation of porcine immune globulin G during postnatal development
- PMID: 38698868
- PMCID: PMC11063267
- DOI: 10.3389/fimmu.2024.1361240
Changes in the N-glycosylation of porcine immune globulin G during postnatal development
Abstract
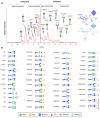
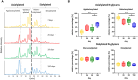
N-glycosylation influences the effectiveness of immune globulin G (IgG) and thus the immunological downstream responses of immune cells. This impact arises from the presence of N-glycans within the Fc region, which not only alters the conformation of IgG but also influences its steric hindrance. Consequently, these modifications affect the interaction between IgG and its binding partners within the immune system. Moreover, this posttranslational modification vary according to the physiological condition of each individual. In this study, we examined the N-glycosylation of IgG in pigs from birth to five months of age. Our analysis identified a total of 48 distinct N-glycan structures. Remarkably, we observed defined changes in the composition of these N-glycans during postnatal development. The presence of agalactosylated and sialylated structures increases in relation to the number of N-glycans terminated by galactose residues during the first months of life. This shift may indicate a transition from passively transferred antibodies from the colostrum of the sow to the active production of endogenous IgG by the pig's own immune system.
Keywords: N-glycan; antibody; glycosylation; ontogenesis; pig; porcine IgG.
Copyright © 2024 Zlatina, Isernhagen, Galuska, Murani and Galuska.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




Similar articles
-
Metabolic control of recombinant monoclonal antibody N-glycosylation in GS-NS0 cells.Biotechnol Bioeng. 2001 Oct 20;75(2):239-51. doi: 10.1002/bit.10022. Biotechnol Bioeng. 2001. PMID: 11536148
-
Measurement of Neutral and Sialylated IgG N-Glycome at Asn-297 by CE-LIF to Assess Hypogalactosylation in Rheumatoid Arthritis.Methods Mol Biol. 2019;1972:77-93. doi: 10.1007/978-1-4939-9213-3_6. Methods Mol Biol. 2019. PMID: 30847785
-
T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies.J Clin Invest. 2013 Sep;123(9):3788-96. doi: 10.1172/JCI65938. Epub 2013 Aug 27. J Clin Invest. 2013. PMID: 23979161 Free PMC article.
-
IgG N-glycans.Adv Clin Chem. 2021;105:1-47. doi: 10.1016/bs.acc.2021.02.001. Epub 2021 Mar 18. Adv Clin Chem. 2021. PMID: 34809825 Review.
-
N-glycomic biomarkers of biological aging and longevity: a link with inflammaging.Ageing Res Rev. 2013 Mar;12(2):685-98. doi: 10.1016/j.arr.2012.02.002. Epub 2012 Feb 14. Ageing Res Rev. 2013. PMID: 22353383 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

