Acid α-glucosidase (GAA) activity and glycogen content in muscle biopsy specimens of patients with Pompe disease: A systematic review
- PMID: 38698877
- PMCID: PMC11064613
- DOI: 10.1016/j.ymgmr.2024.101085
Acid α-glucosidase (GAA) activity and glycogen content in muscle biopsy specimens of patients with Pompe disease: A systematic review
Abstract
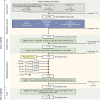
Pompe disease is a rare genetic disorder characterized by a deficiency of acid α-glucosidase (GAA), leading to the accumulation of glycogen in various tissues, especially in skeletal muscles. The disease manifests as a large spectrum of phenotypes from infantile-onset Pompe disease (IOPD) to late-onset Pompe disease (LOPD), depending on the age of symptoms onset. Quantifying GAA activity and glycogen content in skeletal muscle provides important information about the disease severity. However, the distribution of GAA and glycogen levels in skeletal muscles from healthy individuals and those impacted by Pompe disease remains poorly understood, and there is currently no universally accepted standard assay for GAA activity measurement. This systematic literature review aims to provide an overview of the available information on GAA activity and glycogen content levels in skeletal muscle biopsies from patients with Pompe disease. A structured review of PubMed and Google Scholar literature (with the latter used to check that no additional publications were identified) was conducted to identify peer-reviewed publications on glycogen storage disease type II [MeSH term] + GAA, protein human (supplementary concept), Pompe, muscle; and muscle, acid alpha-glucosidase. A limit of English language was applied. Results were grouped by methodologies used to quantify GAA activity and glycogen content in skeletal muscle. The search and selection strategy were devised and carried out in line with Preferred Reporting of Items in Systematic Reviews and Meta-Analysis guidelines and documented using a flowchart. Bibliographies of papers included in the analysis were reviewed and applicable publications not already identified in the search were included. Of the 158 articles retrieved, 24 (comprising >100 muscle biopsies from >100 patients) were included in the analysis, with four different assays. Analysis revealed that patients with IOPD exhibited markedly lower GAA activity in skeletal muscles than those with LOPD, regardless of the measurement method employed. Additionally, patients with IOPD had notably higher glycogen content levels in skeletal muscles than those with LOPD. In general, however, it was difficult to fully characterize GAA activity because of the different methods used. The findings underscore the challenges in the interpretation and comparison of the results across studies because of the substantial methodological variations. There is a need to establish standardized reference ranges of GAA activity and glycogen content in healthy individuals and in Pompe disease patients based on globally standardized methods to improve comparability and reliability in assessing this rare disease.
Keywords: Acid α-glucosidase; Glycogen; Pompe disease; Skeletal muscle biopsy.
© 2024 Published by Elsevier Inc.
Conflict of interest statement
B. Schoser reports: advisory board: Amicus, Astellas, Bayer, Maze, Sanofi, Taysha. Unrestricted Contracted Research; Amicus, Astellas. Honoraria; Kedrion, Alexion, Argenx, Spark. Travel Expenses; Astellas, Amicus, Sanofi. N. Raben is an employee of M6P Therapeutics. F. Varfaj reports no competing interests. M. Walzer is an employee of Astellas Gene Therapies. A. Toscano reports no competing interests.
Figures
Similar articles
-
A molecular analysis of the GAA gene and clinical spectrum in 38 patients with Pompe disease in Japan.Mol Genet Metab Rep. 2017 Oct 31;14:3-9. doi: 10.1016/j.ymgmr.2017.10.009. eCollection 2018 Mar. Mol Genet Metab Rep. 2017. PMID: 29124014 Free PMC article.
-
Remarkably low fibroblast acid α-glucosidase activity in three adults with Pompe disease.Mol Genet Metab. 2012 Nov;107(3):485-9. doi: 10.1016/j.ymgme.2012.09.003. Epub 2012 Sep 7. Mol Genet Metab. 2012. PMID: 23000108
-
Newborn screening for Pompe disease in Italy: Long-term results and future challenges.Mol Genet Metab Rep. 2022 Oct 22;33:100929. doi: 10.1016/j.ymgmr.2022.100929. eCollection 2022 Dec. Mol Genet Metab Rep. 2022. PMID: 36310651 Free PMC article.
-
Gene Therapy for Pompe Disease: The Time is now.Hum Gene Ther. 2019 Oct;30(10):1245-1262. doi: 10.1089/hum.2019.109. Epub 2019 Sep 9. Hum Gene Ther. 2019. PMID: 31298581 Review.
-
Global variations in diagnostic methods and epidemiological estimates in Pompe disease: findings from a scoping review.Orphanet J Rare Dis. 2025 May 6;20(1):216. doi: 10.1186/s13023-025-03679-3. Orphanet J Rare Dis. 2025. PMID: 40329343 Free PMC article.
Cited by
-
Case Report: Incidental late-onset Pompe disease diagnosis in a man with no clinical and instrumental evidence of neuromuscular dysfunction.Front Genet. 2025 Jun 23;16:1574381. doi: 10.3389/fgene.2025.1574381. eCollection 2025. Front Genet. 2025. PMID: 40626179 Free PMC article.
-
Cardiovascular involvement in glycogen storage diseases.Nat Rev Cardiol. 2025 Jun 5. doi: 10.1038/s41569-025-01171-w. Online ahead of print. Nat Rev Cardiol. 2025. PMID: 40473899 Review.
-
Clinical and therapeutic clues from a long-term follow-up: a single center experience on a large LOPD population.J Neurol. 2025 Jun 16;272(7):464. doi: 10.1007/s00415-025-13105-0. J Neurol. 2025. PMID: 40524065
-
Highlights of Precision Medicine, Genetics, Epigenetics and Artificial Intelligence in Pompe Disease.Int J Mol Sci. 2025 Jan 17;26(2):757. doi: 10.3390/ijms26020757. Int J Mol Sci. 2025. PMID: 39859472 Free PMC article. Review.
References
-
- Güngör D., de Vries J.M., Hop W.C., Reuser A.J., van Doorn P.A., van der Ploeg A.T., Hagemans M.L. Survival and associated factors in 268 adults with Pompe disease prior to treatment with enzyme replacement therapy. Orphanet J. Rare Dis. 2011;6:34. https://pubmed.ncbi.nlm.nih.gov/21631931/ - PMC - PubMed
-
- Hagemans M.L., Winkel L.P., Van Doorn P.A., Hop W.J., Loonen M.C., Reuser A.J., Van der Ploeg A.T. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain. 2005;128:671–677. https://pubmed.ncbi.nlm.nih.gov/15659425/ - PubMed
-
- Montagnese F., Barca E., Musumeci O., Mondello S., Migliorato A., Ciranni A., Rodolico C., De Filippi P., Danesino C., Toscano A. Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): unusual features and response to treatment. J. Neurol. 2015;262:968–978. https://pubmed.ncbi.nlm.nih.gov/25673129/ - PubMed
-
- A. Schüller, S. Wenninger, N. Strigl-Pill, B. Schoser, Toward deconstructing the phenotype of late-onset Pompe disease, Am. J. Med. Genet. C: Semin. Med. Genet. 160c (2012) 80–88. https://pubmed.ncbi.nlm.nih.gov/22253010/. - PubMed
-
- van der Beek N.A., van Capelle C.I., van der Velden-van Etten K.I., Hop W.C., van den Berg B., Reuser A.J., van Doorn P.A., van der Ploeg A.T., Stam H. Rate of progression and predictive factors for pulmonary outcome in children and adults with Pompe disease. Mol. Genet. Metab. 2011;104:129–136. https://pubmed.ncbi.nlm.nih.gov/21763167/ - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous