Conductive and alignment-optimized porous fiber conduits with electrical stimulation for peripheral nerve regeneration
- PMID: 38698883
- PMCID: PMC11063606
- DOI: 10.1016/j.mtbio.2024.101064
Conductive and alignment-optimized porous fiber conduits with electrical stimulation for peripheral nerve regeneration
Abstract
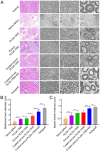
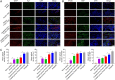
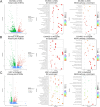
Autologous nerve transplantation (ANT) is currently considered the gold standard for treating long-distance peripheral nerve defects. However, several challenges associated with ANT, such as limited availability of donors, donor site injury, mismatched nerve diameters, and local neuroma formation, remain unresolved. To address these issues comprehensively, we have developed porous poly(lactic-co-glycolic acid) (PLGA) electrospinning fiber nerve guide conduits (NGCs) that are optimized in terms of alignment and conductive coating to facilitate peripheral nerve regeneration (PNR) under electrical stimulation (ES). The physicochemical and biological properties of aligned porous PLGA fibers and poly(3,4-ethylenedioxythiophene):polystyrene sodium sulfonate (PEDOT:PSS) coatings were characterized through assessments of electrical conductivity, surface morphology, mechanical properties, hydrophilicity, and cell proliferation. Material degradation experiments demonstrated the biocompatibility in vivo of electrospinning fiber films with conductive coatings. The conductive NGCs combined with ES effectively facilitated nerve regeneration. The designed porous aligned NGCs with conductive coatings exhibited suitable physicochemical properties and excellent biocompatibility, thereby significantly enhancing PNR when combined with ES. This combination of porous aligned NGCs with conductive coatings and ES holds great promise for applications in the field of PNR.
Keywords: Conductive coating; Electrical stimulation; Electrospinning fibers; Nerve guide conduit; Peripheral nerve defect.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
From innovation to clinic: Emerging strategies harnessing electrically conductive polymers to enhance electrically stimulated peripheral nerve repair.Mater Today Bio. 2024 Dec 19;30:101415. doi: 10.1016/j.mtbio.2024.101415. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39816667 Free PMC article.
-
Conductive MeCbl/PEDOT:PSS/HA hydrogels with electrical stimulation for enhanced peripheral nerve regeneration.Mater Today Bio. 2025 Apr 10;32:101755. doi: 10.1016/j.mtbio.2025.101755. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40290882 Free PMC article.
-
3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair.Front Bioeng Biotechnol. 2019 Oct 16;7:266. doi: 10.3389/fbioe.2019.00266. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31750293 Free PMC article.
-
Peripheral nerve injury repair by electrical stimulation combined with graphene-based scaffolds.Front Bioeng Biotechnol. 2024 Feb 28;12:1345163. doi: 10.3389/fbioe.2024.1345163. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38481574 Free PMC article. Review.
-
Review on electrically conductive smart nerve guide conduit for peripheral nerve regeneration.J Biomed Mater Res A. 2023 Dec;111(12):1916-1950. doi: 10.1002/jbm.a.37595. Epub 2023 Aug 9. J Biomed Mater Res A. 2023. PMID: 37555548 Review.
Cited by
-
Engineering cell-derived extracellular matrix for peripheral nerve regeneration.Mater Today Bio. 2024 Jun 13;27:101125. doi: 10.1016/j.mtbio.2024.101125. eCollection 2024 Aug. Mater Today Bio. 2024. PMID: 38979129 Free PMC article. Review.
-
From innovation to clinic: Emerging strategies harnessing electrically conductive polymers to enhance electrically stimulated peripheral nerve repair.Mater Today Bio. 2024 Dec 19;30:101415. doi: 10.1016/j.mtbio.2024.101415. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39816667 Free PMC article.
-
Biohacking Nerve Repair: Novel Biomaterials, Local Drug Delivery, Electrical Stimulation, and Allografts to Aid Surgical Repair.Bioengineering (Basel). 2024 Jul 31;11(8):776. doi: 10.3390/bioengineering11080776. Bioengineering (Basel). 2024. PMID: 39199733 Free PMC article. Review.
-
Advances in biomaterial-based tissue engineering for peripheral nerve injury repair.Bioact Mater. 2024 Dec 13;46:150-172. doi: 10.1016/j.bioactmat.2024.12.005. eCollection 2025 Apr. Bioact Mater. 2024. PMID: 39760068 Free PMC article. Review.
-
Interlacing biology and engineering: An introduction to textiles and their application in tissue engineering.Mater Today Bio. 2025 Feb 25;31:101617. doi: 10.1016/j.mtbio.2025.101617. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40124339 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

