Expression of Toll-like receptors in the cerebellum during pathogenesis of prion disease
- PMID: 38698886
- PMCID: PMC11063360
- DOI: 10.3389/fnbeh.2024.1341901
Expression of Toll-like receptors in the cerebellum during pathogenesis of prion disease
Abstract
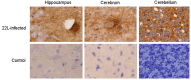
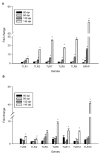
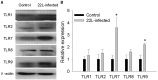
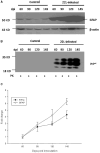
Prion diseases, such as scrapie, entail the accumulation of disease-specific prion protein (PrPSc) within the brain. Toll-like receptors (TLRs) are crucial components of the pattern recognition system. They recognize pathogen-associated molecular patterns (PAMPs) and play a central role in orchestrating host innate immune responses. The expression levels of Toll-like receptors (TLRs) in the central nervous system (CNS) were not well-defined. To establish a model of prion diseases in BALB/C mice, the 22L strain was employed. The features of the 22L strain were analyzed, and the cerebellum exhibited severe pathological changes. TLR1-13 levels in the cerebellum were measured using quantitative polymerase chain reaction (qPCR) at time points of 60, 90, 120, and the final end point (145 days post-infection). During the pathogenesis, the expression levels of Toll-like receptors (TLRs) 1, 2, 7, 8, and 9 increased in a time-dependent manner. This trend mirrored the expression patterns of PrPSc (the pathological isoform of the prion protein) and glial fibrillary acidic protein. Notably, at the end point, TLR1-13 levels were significantly elevated. Protein level of TLR7 and TLR9 showed increasing at the end point of the 22L-infected mice. A deeper understanding of the increased Toll-like receptors (TLRs) in prion diseases could shed light on their role in initiating immune responses at various stages during pathogenesis. This insight is particularly relevant when considering TLRs as potential therapeutic targets for prion diseases.
Keywords: PrPSc; Toll-like receptor; central nervous system; innate immune; prion disease.
Copyright © 2024 Liao, Zhu, Liao, Liu, Hou and Wan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Distinctive Toll-like Receptors Gene Expression and Glial Response in Different Brain Regions of Natural Scrapie.Int J Mol Sci. 2022 Mar 25;23(7):3579. doi: 10.3390/ijms23073579. Int J Mol Sci. 2022. PMID: 35408945 Free PMC article.
-
Accelerated prion disease pathogenesis in Toll-like receptor 4 signaling-mutant mice.J Virol. 2008 Nov;82(21):10701-8. doi: 10.1128/JVI.00522-08. Epub 2008 Aug 20. J Virol. 2008. PMID: 18715916 Free PMC article.
-
Prion Protein Devoid of the Octapeptide Repeat Region Delays Bovine Spongiform Encephalopathy Pathogenesis in Mice.J Virol. 2017 Dec 14;92(1):e01368-17. doi: 10.1128/JVI.01368-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046443 Free PMC article.
-
Novel mechanisms of degeneration of the central nervous system--prion structure and biology.Ciba Found Symp. 1988;135:239-60. doi: 10.1002/9780470513613.ch16. Ciba Found Symp. 1988. PMID: 2900720 Review.
-
Synaptic pathology and cell death in the cerebellum in Creutzfeldt-Jakob disease.Cerebellum. 2002 Jul;1(3):213-22. doi: 10.1080/14734220260418448. Cerebellum. 2002. PMID: 12879983 Review.
Cited by
-
Heavy Metal Interactions with Neuroglia and Gut Microbiota: Implications for Huntington's Disease.Cells. 2024 Jul 3;13(13):1144. doi: 10.3390/cells13131144. Cells. 2024. PMID: 38994995 Free PMC article. Review.
References
-
- Balamuralidhara V. (2023). Transmissible spongiform encephalopathy and its regulations. Ind. J. Pharm. Edu. Res, 57(1s), s7–s12. 10.5530/ijper.57.1s.2 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

