Relative Vaccine Effectiveness of Cell- vs Egg-Based Quadrivalent Influenza Vaccine Against Test-Confirmed Influenza Over 3 Seasons Between 2017 and 2020 in the United States
- PMID: 38698895
- PMCID: PMC11064727
- DOI: 10.1093/ofid/ofae175
Relative Vaccine Effectiveness of Cell- vs Egg-Based Quadrivalent Influenza Vaccine Against Test-Confirmed Influenza Over 3 Seasons Between 2017 and 2020 in the United States
Abstract
Background: Influenza vaccine viruses grown in eggs may acquire egg-adaptive mutations that may reduce antigenic similarity between vaccine and circulating influenza viruses and decrease vaccine effectiveness. We compared cell- and egg-based quadrivalent influenza vaccines (QIVc and QIVe, respectively) for preventing test-confirmed influenza over 3 US influenza seasons (2017-2020).
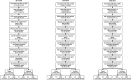
Methods: Using a retrospective test-negative design, we estimated the relative vaccine effectiveness (rVE) of QIVc vs QIVe among individuals aged 4 to 64 years who had an acute respiratory or febrile illness and were tested for influenza in routine outpatient care. Exposure, outcome, and covariate data were obtained from electronic health records linked to pharmacy and medical claims. Season-specific rVE was estimated by comparing the odds of testing positive for influenza among QIVc vs QIVe recipients. Models were adjusted for age, sex, geographic region, influenza test date, and additional unbalanced covariates. A doubly robust approach was used combining inverse probability of treatment weights with multivariable regression.
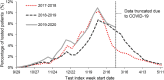
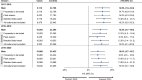
Results: The study included 31 824, 33 388, and 34 398 patients in the 2017-2018, 2018-2019, and 2019-2020 seasons, respectively; ∼10% received QIVc and ∼90% received QIVe. QIVc demonstrated superior effectiveness vs QIVe in prevention of test-confirmed influenza: rVEs were 14.8% (95% CI, 7.0%-22.0%) in 2017-2018, 12.5% (95% CI, 4.7%-19.6%) in 2018-2019, and 10.0% (95% CI, 2.7%-16.7%) in 2019-2020.
Conclusions: This study demonstrated consistently superior effectiveness of QIVc vs QIVe in preventing test-confirmed influenza over 3 seasons characterized by different circulating viruses and degrees of egg adaptation.
Keywords: cell-based quadrivalent influenza vaccine; egg adaptation; influenza; influenza virus mismatch; relative vaccine effectiveness.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. N. S., I. M., and M. D. M. H. are employees of CSL Seqirus. C. W. M., K. W. M., A. D., and A. N. B. are employees of Veradigm. S. G. S. reports receipt of consulting fees for CSL Seqirus, Pfizer, Moderna, and EvoHealth.
Figures


Similar articles
-
A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017-18 influenza season.Vaccine. 2020 Sep 11;38(40):6334-6343. doi: 10.1016/j.vaccine.2020.07.023. Epub 2020 Jul 30. Vaccine. 2020. PMID: 32739119
-
Cell-based influenza vaccines: An effective vaccine option for under 60-year-olds.GMS Hyg Infect Control. 2024 Apr 30;19:Doc21. doi: 10.3205/dgkh000476. eCollection 2024. GMS Hyg Infect Control. 2024. PMID: 38766639 Free PMC article. Review.
-
Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018-19 Influenza Season in the United States.Vaccines (Basel). 2021 Jan 23;9(2):80. doi: 10.3390/vaccines9020080. Vaccines (Basel). 2021. PMID: 33498724 Free PMC article.
-
Effectiveness of Cell-Based Quadrivalent Seasonal Influenza Vaccine: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2023 Oct 17;11(10):1607. doi: 10.3390/vaccines11101607. Vaccines (Basel). 2023. PMID: 37897009 Free PMC article. Review.
-
Cost-effectiveness of the cell-based quadrivalent versus the standard egg-based quadrivalent influenza vaccine in Germany.J Med Econ. 2021 Jan-Dec;24(1):490-501. doi: 10.1080/13696998.2021.1908000. J Med Econ. 2021. PMID: 33761803
Cited by
-
Cell-Cultured Influenza Vaccine Enhances IFN-γ+ T Cell and Memory T Cell Responses Following A/Victoria/2570/2019 IVR-215 (A/H1N1) Infection.Vaccines (Basel). 2024 Dec 11;12(12):1392. doi: 10.3390/vaccines12121392. Vaccines (Basel). 2024. PMID: 39772053 Free PMC article.
-
Cost-Effectiveness of Adjuvanted Influenza Vaccine Compared with Standard and High-Dose Influenza Vaccines for Persons Aged ≥50 Years in Spain.Vaccines (Basel). 2025 Mar 19;13(3):323. doi: 10.3390/vaccines13030323. Vaccines (Basel). 2025. PMID: 40266221 Free PMC article.
References
-
- Centers for Disease Control and Prevention . Disease burden of flu. 2022. Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed 5 October 2023.

