Tissue-specific localization of tick-borne pathogens in ticks collected from camels in Kenya: insights into vector competence
- PMID: 38698904
- PMCID: PMC11063324
- DOI: 10.3389/fcimb.2024.1382228
Tissue-specific localization of tick-borne pathogens in ticks collected from camels in Kenya: insights into vector competence
Abstract
Background: Tick-borne pathogen (TBP) surveillance studies often use whole-tick homogenates when inferring tick-pathogen associations. However, localized TBP infections within tick tissues (saliva, hemolymph, salivary glands, and midgut) can inform pathogen transmission mechanisms and are key to disentangling pathogen detection from vector competence.
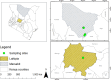
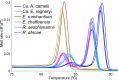
Methods: We screened 278 camel blood samples and 504 tick tissue samples derived from 126 camel ticks sampled in two Kenyan counties (Laikipia and Marsabit) for Anaplasma, Ehrlichia, Coxiella, Rickettsia, Theileria, and Babesia by PCR-HRM analysis.
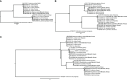
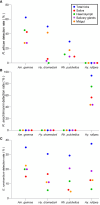
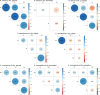
Results: Candidatus Anaplasma camelii infections were common in camels (91%), but absent in all samples from Rhipicephalus pulchellus, Amblyomma gemma, Hyalomma dromedarii, and Hyalomma rufipes ticks. We detected Ehrlichia ruminantium in all tissues of the four tick species, but Rickettsia aeschlimannii was only found in Hy. rufipes (all tissues). Rickettsia africae was highest in Am. gemma (62.5%), mainly in the hemolymph (45%) and less frequently in the midgut (27.5%) and lowest in Rh. pulchellus (29.4%), where midgut and hemolymph detection rates were 17.6% and 11.8%, respectively. Similarly, in Hy. dromedarii, R. africae was mainly detected in the midgut (41.7%) but was absent in the hemolymph. Rickettsia africae was not detected in Hy. rufipes. No Coxiella, Theileria, or Babesia spp. were detected in this study.
Conclusions: The tissue-specific localization of R. africae, found mainly in the hemolymph of Am. gemma, is congruent with the role of this tick species as its transmission vector. Thus, occurrence of TBPs in the hemolymph could serve as a predictor of vector competence of TBP transmission, especially in comparison to detection rates in the midgut, from which they must cross tissue barriers to effectively replicate and disseminate across tick tissues. Further studies should focus on exploring the distribution of TBPs within tick tissues to enhance knowledge of TBP epidemiology and to distinguish competent vectors from dead-end hosts.
Keywords: Amblyomma gemma; Ehrlichia; Hyalomma dromedarii; Hyalomma rufipes; Rhipicephalus pulchellus; Rickettsia; dromedary camels; tick tissues.
Copyright © 2024 Khogali, Bastos, Bargul, Getange, Kabii, Masiga and Villinger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
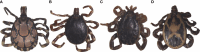

References
-
- Bastos A. D., Mohammed O. B., Bennett N. C., Petevinos C., Alagaili A. N. (2015). Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius). Vet. Microbiol. 179, 310–314. doi: 10.1016/j.vetmic.2015.06.001 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

