Kinetic Studies on the 2-Oxoglutarate/Fe(II)-Dependent Nucleic Acid Modifying Enzymes from the AlkB and TET Families
- PMID: 38698914
- PMCID: PMC11065319
- DOI: 10.3390/dna3020005
Kinetic Studies on the 2-Oxoglutarate/Fe(II)-Dependent Nucleic Acid Modifying Enzymes from the AlkB and TET Families
Abstract
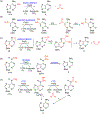
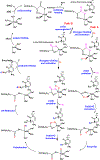
Nucleic acid methylations are important genetic and epigenetic biomarkers. The formation and removal of these markers is related to either methylation or demethylation. In this review, we focus on the demethylation or oxidative modification that is mediated by the 2-oxoglutarate (2-OG)/Fe(II)-dependent AlkB/TET family enzymes. In the catalytic process, most enzymes oxidize 2-OG to succinate, in the meantime oxidizing methyl to hydroxymethyl, leaving formaldehyde and generating demethylated base. The AlkB enzyme from Escherichia coli has nine human homologs (ALKBH1-8 and FTO) and the TET family includes three members, TET1 to 3. Among them, some enzymes have been carefully studied, but for certain enzymes, few studies have been carried out. This review focuses on the kinetic properties of those 2-OG/Fe(II)-dependent enzymes and their alkyl substrates. We also provide some discussions on the future directions of this field.
Keywords: 2-OG-dependent enzyme; ALKBH protein; AlkB; FTO; TET; kinetics.
Conflict of interest statement
Conflicts of Interest: The authors declare no competing financial interests.
Figures
Similar articles
-
Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids.Cell Cycle. 2009 Jun 1;8(11):1698-710. doi: 10.4161/cc.8.11.8580. Epub 2009 Jun 27. Cell Cycle. 2009. PMID: 19411852 Free PMC article.
-
The role of demethylase AlkB homologs in cancer.Front Oncol. 2023 Mar 16;13:1153463. doi: 10.3389/fonc.2023.1153463. eCollection 2023. Front Oncol. 2023. PMID: 37007161 Free PMC article. Review.
-
Non-homologous functions of the AlkB homologs.J Mol Cell Biol. 2015 Dec;7(6):494-504. doi: 10.1093/jmcb/mjv029. Epub 2015 May 23. J Mol Cell Biol. 2015. PMID: 26003568 Review.
-
The AlkB Family of Fe(II)/α-Ketoglutarate-dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond.J Biol Chem. 2015 Aug 21;290(34):20734-20742. doi: 10.1074/jbc.R115.656462. Epub 2015 Jul 7. J Biol Chem. 2015. PMID: 26152727 Free PMC article. Review.
-
The AlkB Family of Fe (II)/Alpha-Ketoglutarate-Dependent Dioxygenases Modulates Embryogenesis through Epigenetic Regulation.Curr Stem Cell Res Ther. 2018;13(2):136-143. doi: 10.2174/1574888X12666171027105532. Curr Stem Cell Res Ther. 2018. PMID: 29076432 Review.
Cited by
-
Interconnections between m6A RNA modification, RNA structure, and protein-RNA complex assembly.Life Sci Alliance. 2023 Nov 7;7(1):e202302240. doi: 10.26508/lsa.202302240. Print 2024 Jan. Life Sci Alliance. 2023. PMID: 37935465 Free PMC article. Review.
-
An α-ketoglutarate conformational switch controls iron accessibility, activation, and substrate selection of the human FTO protein.Proc Natl Acad Sci U S A. 2024 Jun 18;121(25):e2404457121. doi: 10.1073/pnas.2404457121. Epub 2024 Jun 12. Proc Natl Acad Sci U S A. 2024. PMID: 38865275 Free PMC article.
-
3,N4-Etheno-5-methylcytosine blocks TET1-3 oxidation but is repaired by ALKBH2, 3 and FTO.Nucleic Acids Res. 2024 Nov 11;52(20):12378-12389. doi: 10.1093/nar/gkae818. Nucleic Acids Res. 2024. PMID: 39315710 Free PMC article.
References
-
- Mathison BH; Frame SR; Bogdanffy MS DNA Methylation, Cell Proliferation, and Histopathology in Rats Following Repeated Inhalation Exposure to Dimethyl Sulfate. Inhal. Toxicol 2004, 16, 581–592. - PubMed
-
- Marnett L Endogenous DNA Damage and Mutation. Trends Genet. 2001, 17, 214–221. - PubMed
-
- Bordin DL; Lirussi L; Nilsen H Cellular Response to Endogenous DNA Damage: DNA Base Modifications in Gene Expression Regulation. DNA Repair 2021, 99, 103051. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
