An orally administered bacterial membrane protein nanodrug ameliorates doxorubicin cardiotoxicity through alleviating impaired intestinal barrier
- PMID: 38698916
- PMCID: PMC11063951
- DOI: 10.1016/j.bioactmat.2024.03.027
An orally administered bacterial membrane protein nanodrug ameliorates doxorubicin cardiotoxicity through alleviating impaired intestinal barrier
Abstract
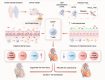
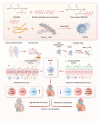
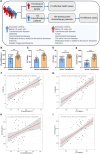
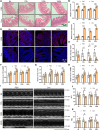
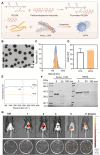
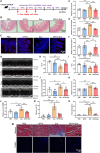
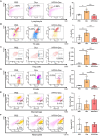
The cardiotoxicity caused by Dox chemotherapy represents a significant limitation to its clinical application and is a major cause of late death in patients undergoing chemotherapy. Currently, there are no effective treatments available. Our analysis of 295 clinical samples from 132 chemotherapy patients and 163 individuals undergoing physical examination revealed a strong positive correlation between intestinal barrier injury and the development of cardiotoxicity in chemotherapy patients. We developed a novel orally available and intestinal targeting protein nanodrug by assembling membrane protein Amuc_1100 (obtained from intestinal bacteria Akkermansia muciniphila), fluorinated polyetherimide, and hyaluronic acid. The protein nanodrug demonstrated favorable stability against hydrolysis compared with free Amuc_1100. The in vivo results demonstrated that the protein nanodrug can alleviate Dox-induced cardiac toxicity by improving gut microbiota, increasing the proportion of short-chain fatty acid-producing bacteria from the Lachnospiraceae family, and further enhancing the levels of butyrate and pentanoic acids, ultimately regulating the homeostasis repair of lymphocytes in the spleen and heart. Therefore, we believe that the integrity of the intestinal barrier plays an important role in the development of chemotherapy-induced cardiotoxicity. Protective interventions targeting the intestinal barrier may hold promise as a general clinical treatment regimen for reducing Dox-induced cardiotoxicity.
Keywords: Doxorubicin cardiotoxicity; Homeostasis of lymphocytes; Intestinal barrier; Oral nanodrugs; Protein delivery.
© 2024 The Authors.
Conflict of interest statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be constructed as influencing the position presented in, or the review of, the manuscript entitled.
Figures



Similar articles
-
The Gut-Heart Axis and Its Role in Doxorubicin-Induced Cardiotoxicity: A Narrative Review.Microorganisms. 2025 Apr 9;13(4):855. doi: 10.3390/microorganisms13040855. Microorganisms. 2025. PMID: 40284691 Free PMC article. Review.
-
Akkermansia muciniphila and its outer membrane protein Amuc_1100 prophylactically attenuate 5-fluorouracil-induced intestinal mucositis.Biochem Biophys Res Commun. 2022 Jul 23;614:34-40. doi: 10.1016/j.bbrc.2022.04.135. Epub 2022 May 3. Biochem Biophys Res Commun. 2022. PMID: 35567942
-
Akkermansia muciniphila and its membrane protein ameliorates intestinal inflammatory stress and promotes epithelial wound healing via CREBH and miR-143/145.J Biomed Sci. 2023 Jun 7;30(1):38. doi: 10.1186/s12929-023-00935-1. J Biomed Sci. 2023. PMID: 37287024 Free PMC article.
-
Zn(ii)-Curcumin supplementation alleviates gut dysbiosis and zinc dyshomeostasis during doxorubicin-induced cardiotoxicity in rats.Food Funct. 2019 Sep 1;10(9):5587-5604. doi: 10.1039/c9fo01034c. Epub 2019 Aug 21. Food Funct. 2019. PMID: 31432062
-
Targeting mitochondrial dynamics proteins for the treatment of doxorubicin-induced cardiotoxicity.Front Mol Biosci. 2023 Aug 3;10:1241225. doi: 10.3389/fmolb.2023.1241225. eCollection 2023. Front Mol Biosci. 2023. PMID: 37602332 Free PMC article. Review.
Cited by
-
Engineered bacterial membrane biomimetic covalent organic framework as nano-immunopotentiator for cancer immunotherapy.Bioact Mater. 2025 Jan 25;47:283-294. doi: 10.1016/j.bioactmat.2025.01.018. eCollection 2025 May. Bioact Mater. 2025. PMID: 39925708 Free PMC article.
-
Sulfhydrated albumin transmits H2S signaling and ameliorates DOX-induced multiorgan injuries.Redox Biol. 2025 Jun;83:103631. doi: 10.1016/j.redox.2025.103631. Epub 2025 Apr 8. Redox Biol. 2025. PMID: 40228337 Free PMC article.
-
Big lessons from the little Akkermansia muciniphila in hepatocellular carcinoma.Front Immunol. 2025 Feb 14;16:1524563. doi: 10.3389/fimmu.2025.1524563. eCollection 2025. Front Immunol. 2025. PMID: 40028328 Free PMC article. Review.
-
The Gut-Heart Axis and Its Role in Doxorubicin-Induced Cardiotoxicity: A Narrative Review.Microorganisms. 2025 Apr 9;13(4):855. doi: 10.3390/microorganisms13040855. Microorganisms. 2025. PMID: 40284691 Free PMC article. Review.
References
-
- Beavers C.J., Rodgers J.E., Bagnola A.J., Beckie T.M., Campia U., Di Palo K.E., Okwuosa T.M., Przespolewski E.R., Dent S., American Heart Association Clinical Pharmacology C. Cardio-Oncology Committee of the Council on Clinical, C., Council on, G.; precision, M.; the council on peripheral vascular, D., cardio-oncology drug interactions: a scientific statement from the American heart association. Circulation. 2022;145(15):e811–e838. - PubMed
-
- Sawicki K.T., Sala V., Prever L., Hirsch E., Ardehali H., Ghigo A. Preventing and treating anthracycline cardiotoxicity: new insights. Annu. Rev. Pharmacol. Toxicol. 2021;61:309–332. - PubMed
-
- Gulati G., Heck S.L., Ree A.H., Hoffmann P., Schulz-Menger J., Fagerland M.W., Gravdehaug B., von Knobelsdorff-Brenkenhoff F., Bratland A., Storas T.H., Hagve T.A., Rosjo H., Steine K., Geisler J., Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016;37(21):1671–1680. - PMC - PubMed
-
- Lipshultz S.E., Cochran T.R., Franco V.I., Miller T.L. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. 2013;10(12):697–710. - PubMed
LinkOut - more resources
Full Text Sources

