Ultrastructural 3D Microscopy for Biomedicine: Principles, Applications, and Perspectives
- PMID: 38698961
- PMCID: PMC11062107
- DOI: 10.32607/actanaturae.27323
Ultrastructural 3D Microscopy for Biomedicine: Principles, Applications, and Perspectives
Abstract
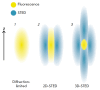
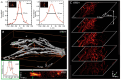
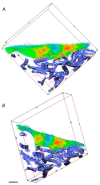
Modern biomedical research often requires a three-dimensional microscopic analysis of the ultrastructure of biological objects and materials. Conceptual technical and methodological solutions for three-dimensional structure reconstruction are needed to improve the conventional optical, electron, and probe microscopy methods, which to begin with allow one to obtain two-dimensional images and data. This review discusses the principles and potential applications of such techniques as serial section transmission electron microscopy; techniques based on scanning electron microscopy (SEM) (array tomography, focused ion beam SEM, and serial block-face SEM). 3D analysis techniques based on modern super-resolution optical microscopy methods are described (stochastic optical reconstruction microscopy and stimulated emission depletion microscopy), as well as ultrastructural 3D microscopy methods based on scanning probe microscopy and the feasibility of combining them with optical techniques. A comparative analysis of the advantages and shortcomings of the discussed approaches is performed.
Keywords: biomedical research; electron microscopy; scanning probe microscopy; super-resolution optical microscopy; tomography; ultrastructural 3D microscopy.
Copyright ® 2024 National Research University Higher School of Economics.
Figures













Similar articles
-
The application and development of electron microscopy for three-dimensional reconstruction in life science: a review.Cell Tissue Res. 2024 Apr;396(1):1-18. doi: 10.1007/s00441-024-03878-7. Epub 2024 Feb 28. Cell Tissue Res. 2024. PMID: 38416172 Review.
-
Applications of Scanning Electron Microscopy Using Secondary and Backscattered Electron Signals in Neural Structure.Front Neuroanat. 2021 Dec 2;15:759804. doi: 10.3389/fnana.2021.759804. eCollection 2021. Front Neuroanat. 2021. PMID: 34955763 Free PMC article.
-
Recent advances in electron microscopy for the diagnosis and research of glomerular diseases.Kidney Res Clin Pract. 2023 Mar;42(2):155-165. doi: 10.23876/j.krcp.21.270. Epub 2022 May 4. Kidney Res Clin Pract. 2023. PMID: 35545227 Free PMC article.
-
A workflow for 3D-CLEM investigating liver tissue.J Microsc. 2021 Mar;281(3):231-242. doi: 10.1111/jmi.12967. Epub 2020 Oct 27. J Microsc. 2021. PMID: 33034376
-
Three dimensional reconstruction by electron microscopy in the life sciences: An introduction for cell and tissue biologists.Mol Reprod Dev. 2015 Jul-Aug;82(7-8):530-47. doi: 10.1002/mrd.22455. Epub 2015 Feb 4. Mol Reprod Dev. 2015. PMID: 25652003 Review.
References
-
- Franken L.E., Grünewald K., Boekema E.J., Stuart M.C.A.. Small. 2020;16(14):e1906198. - PubMed
-
- Northover A.S., Keatley S., Elliot A.D., Hobbs R.P., Yang R., Lymbery A.J., Godfrey S.S., Wayne A.F., Thompson R.C.A.. Syst. Parasitol. 2019;96(7):553–563. - PubMed
-
- Bian K., Gerber C., Heinrich A., Müller D., Scheuring S., Jiang Y., Nat. Rev. Meth. Primers. 2021;1(1):1–36.:10.1038/s43586-021-00033-2.
-
- Hell S.W., Wichmann J.. Opt. Lett. 1994;19(11):780–782. - PubMed
LinkOut - more resources
Full Text Sources
