High pathogenic avian influenza A(H5) viruses of clade 2.3.4.4b in Europe-Why trends of virus evolution are more difficult to predict
- PMID: 38699215
- PMCID: PMC11065109
- DOI: 10.1093/ve/veae027
High pathogenic avian influenza A(H5) viruses of clade 2.3.4.4b in Europe-Why trends of virus evolution are more difficult to predict
Abstract
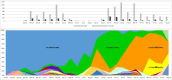
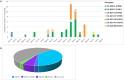
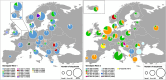
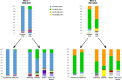
Since 2016, A(H5Nx) high pathogenic avian influenza (HPAI) virus of clade 2.3.4.4b has become one of the most serious global threats not only to wild and domestic birds, but also to public health. In recent years, important changes in the ecology, epidemiology, and evolution of this virus have been reported, with an unprecedented global diffusion and variety of affected birds and mammalian species. After the two consecutive and devastating epidemic waves in Europe in 2020-2021 and 2021-2022, with the second one recognized as one of the largest epidemics recorded so far, this clade has begun to circulate endemically in European wild bird populations. This study used the complete genomes of 1,956 European HPAI A(H5Nx) viruses to investigate the virus evolution during this varying epidemiological outline. We investigated the spatiotemporal patterns of A(H5Nx) virus diffusion to/from and within Europe during the 2020-2021 and 2021-2022 epidemic waves, providing evidence of ongoing changes in transmission dynamics and disease epidemiology. We demonstrated the high genetic diversity of the circulating viruses, which have undergone frequent reassortment events, providing for the first time a complete overview and a proposed nomenclature of the multiple genotypes circulating in Europe in 2020-2022. We described the emergence of a new genotype with gull adapted genes, which offered the virus the opportunity to occupy new ecological niches, driving the disease endemicity in the European wild bird population. The high propensity of the virus for reassortment, its jumps to a progressively wider number of host species, including mammals, and the rapid acquisition of adaptive mutations make the trend of virus evolution and spread difficult to predict in this unfailing evolving scenario.
Keywords: Europe; high pathogenic avian influenza A(H5) viruses; phylodynamics; reassortments; spatial spread.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures



References
-
- Adams S. E. et al. (2019) ‘Effect of Influenza H1N1 Neuraminidase V116A and I117V Mutations on NA Activity and Sensitivity to NA Inhibitors’, Antiviral Research, 169: 104539. - PubMed

