Lanthanide MOF-based luminescent sensor arrays for the detection of castration-resistant prostate cancer curing drugs and biomarkers
- PMID: 38699260
- PMCID: PMC11062119
- DOI: 10.1039/d3sc06899d
Lanthanide MOF-based luminescent sensor arrays for the detection of castration-resistant prostate cancer curing drugs and biomarkers
Abstract
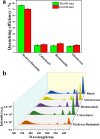
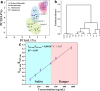
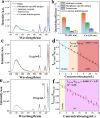
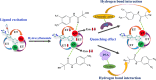
In recent years, castration-resistant prostate cancer (CRPC) has profoundly impacted the lives of many men, and early diagnosis of medication and illness is crucial. Therefore, a highly efficient detection method for CRPC biomarkers and curing drugs is required. However, the complex and diverse structures of CRPC drugs pose significant challenges for their detection and differentiation. Lanthanide metal-organic frameworks (Ln-MOFs) show great potential for sensing applications due to their intense and characteristic luminescence. In this work, a series of new bimetallic Ln-MOFs (EuxTb1-x-MOF) based luminescent sensor arrays have been developed to identify CRPC drugs, including in mixtures, via principal component analysis (PCA) and hierarchical cluster analysis (HCA) methods. These Ln-MOFs are built with a highly conjugated H2L linker (H2L = 5-(4-(triazole-1-yl)phenyl)isophthalic acid) and exhibit robust strong luminescence emissions (mainly located at 543 and 614 nm) and high energy transfer efficiencies. More specifically, Eu0.096Tb0.904-MOF (MOF 3) has demonstrated good sensing performances for CRPC curing drugs in real human serum samples. Furthermore, the curing drug hydroxyflutamide has been combined with MOF 3, to construct a robust composite sensing platform MOF 3@hydroxyflutamide for highly efficient detection of CRPC biomarkers such as the androgen receptor (AR) and prostate-specific antigen (PSA). Finally, luminescence lifetime measurements, zeta potential measurements, and density functional theory (DFT) calculations were performed to gain insights into the sensing mechanism.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
A MOF/DNA luminescent sensing platform for detection of potential COVID-19 biomarkers and drugs.Chem Sci. 2023 Apr 19;14(20):5386-5395. doi: 10.1039/d3sc00106g. eCollection 2023 May 24. Chem Sci. 2023. PMID: 37234896 Free PMC article.
-
Ln-MOF Based Ratiometric Luminescent Sensor for the Detection of Potential COVID-19 Drugs.Chemistry. 2023 Feb 24;29(12):e202203136. doi: 10.1002/chem.202203136. Epub 2023 Jan 13. Chemistry. 2023. PMID: 36424358
-
Lanthanide-Functionalized Metal-Organic Framework Hybrid Systems To Create Multiple Luminescent Centers for Chemical Sensing.Acc Chem Res. 2017 Nov 21;50(11):2789-2798. doi: 10.1021/acs.accounts.7b00387. Epub 2017 Oct 6. Acc Chem Res. 2017. PMID: 28984437
-
The luminescent principle and sensing mechanism of metal-organic framework for bioanalysis and bioimaging.Biosens Bioelectron. 2024 Apr 1;249:116008. doi: 10.1016/j.bios.2024.116008. Epub 2024 Jan 18. Biosens Bioelectron. 2024. PMID: 38245932 Review.
-
Encapsulation of Luminescent Guests to Construct Luminescent Metal-Organic Frameworks for Chemical Sensing.ACS Sens. 2021 Mar 26;6(3):641-658. doi: 10.1021/acssensors.0c02562. Epub 2021 Feb 11. ACS Sens. 2021. PMID: 33571406 Review.
Cited by
-
In situ growth of luminescent d-f MOF nanostructures on bacterial cellulose as an accessible kit for early jaundice diagnosis.Sci Rep. 2025 May 9;15(1):16216. doi: 10.1038/s41598-025-94147-2. Sci Rep. 2025. PMID: 40346306 Free PMC article.
-
Self-supplying coreactant radical and structural distortion induced by carbonate ligand in metal-organic framework for anomalous deep-red Self-electrochemiluminescence.Chem Sci. 2025 Jun 10;16(27):12493-12498. doi: 10.1039/d5sc02359a. eCollection 2025 Jul 10. Chem Sci. 2025. PMID: 40502819 Free PMC article.
-
Digitonin-Loaded Nanoscale Metal-Organic Framework for Mitochondria-Targeted Radiotherapy-Radiodynamic Therapy and Disulfidptosis.Adv Mater. 2024 Sep 10:e2405494. doi: 10.1002/adma.202405494. Online ahead of print. Adv Mater. 2024. PMID: 39252688
-
A novel Ln3+/Al3+ metallacrown multifunctional material for latent fingerprint detection, luminescent thermometers and luminescent sensors.Chem Sci. 2025 Feb 11;16(11):4821-4830. doi: 10.1039/d4sc08549c. eCollection 2025 Mar 12. Chem Sci. 2025. PMID: 39944122 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

