Photochemical three-component assembly of tri-substituted oxazoles through a carbenic phosphorus-nitrile hybrid ylide formation/trapping cascade
- PMID: 38699275
- PMCID: PMC11062088
- DOI: 10.1039/d4sc01355g
Photochemical three-component assembly of tri-substituted oxazoles through a carbenic phosphorus-nitrile hybrid ylide formation/trapping cascade
Abstract
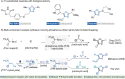
Construction of complex molecular skeletons with ubiquitous chemical feedstocks in a single transformation is highly appealing in organic synthesis. We report a novel visible-light-induced three-component reaction for the construction of complex 2,4,5-trisubstituted oxazoles, which are valuable in medicinal chemistry, from simple and readily available iodonium-phosphonium hybrid ylides, carboxylic acids, and nitriles. This reaction features a carbenic phosphorus-nitrile hybrid ylide formation/trapping cascade, in which a photo-generated α-phosphonium carbene acts as a sequence trigger. This catalyst- and additive-free transformation exhibits high efficiency and broad substrate scope for synthesizing diverse oxazoles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Three-component modular synthesis of chiral 1,3-dioxoles via a Rh-catalyzed carbenic olefination cascade.Chem Sci. 2024 Oct 15;15(44):18564-71. doi: 10.1039/d4sc06166g. Online ahead of print. Chem Sci. 2024. PMID: 39444556 Free PMC article.
-
Visible-Light-Induced Imide Synthesis through a Nitrile Ylide Formation/Trapping Cascade.Org Lett. 2022 Sep 16;24(36):6647-6652. doi: 10.1021/acs.orglett.2c02671. Epub 2022 Sep 2. Org Lett. 2022. PMID: 36053175
-
Catalytic and Base-free Suzuki-type α-Arylation of Cyclic 1,3-Dicarbonyls via a Cyclic Iodonium Ylide Strategy.Angew Chem Int Ed Engl. 2024 Apr 22;63(17):e202400741. doi: 10.1002/anie.202400741. Epub 2024 Mar 19. Angew Chem Int Ed Engl. 2024. PMID: 38385585
-
Nitrile-synthesizing enzymes and biocatalytic synthesis of volatile nitrile compounds: A review.J Biotechnol. 2024 Mar 20;384:20-28. doi: 10.1016/j.jbiotec.2024.02.007. Epub 2024 Feb 21. J Biotechnol. 2024. PMID: 38395363 Review.
-
Carbenes and phosphonium ylides: a fruitful association in coordination chemistry.Dalton Trans. 2021 Nov 23;50(45):16434-16442. doi: 10.1039/d1dt03155d. Dalton Trans. 2021. PMID: 34664574 Review.
Cited by
-
Three-component modular synthesis of chiral 1,3-dioxoles via a Rh-catalyzed carbenic olefination cascade.Chem Sci. 2024 Oct 15;15(44):18564-71. doi: 10.1039/d4sc06166g. Online ahead of print. Chem Sci. 2024. PMID: 39444556 Free PMC article.
-
Synthesis of Oxazoles Containing CF3-Substituted Alcohol Unit via Tandem Cycloisomerization/Hydroxyalkylation from N-Propargylamides with Trifluoropyruvates.Molecules. 2024 Dec 11;29(24):5848. doi: 10.3390/molecules29245848. Molecules. 2024. PMID: 39769937 Free PMC article.
References
-
- Eicher T., Hauptmann S. and Speicher A., The Chemistry of Heterocycles, Wiley-VCH, 2003
- Pozharskii A. F., Soldatenkov A. T. and Katritzky A. R., Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, John Wiley & Sons, 2011
-
- Davyt D. Serra G. Mar. Drugs. 2010;8:2755–2780. doi: 10.3390/md8112755. - DOI - PMC - PubMed
- Jin Z. Nat. Prod. Rep. 2011;28:1143–1191. doi: 10.1039/C0NP00074D. - DOI - PubMed
- Zhang H.-Z. Zhao Z.-L. Zhou C.-H. Eur. J. Med. Chem. 2018;144:444–492. doi: 10.1016/j.ejmech.2017.12.044. - DOI - PubMed
- Kakkar S. Narasimhan B. BMC Chem. 2019;13:16. doi: 10.1186/s13065-019-0531-9. - DOI - PMC - PubMed
- Jaitak V. Kulkarni S. Kaur K. Anti-Cancer Agents Med. Chem. 2022;22:1859–1882. doi: 10.2174/1871520621666210915095421. - DOI - PubMed
-
- Palmer D. C., Oxazoles: Synthesis, Reactions, and Spectroscopy, Part A, John Wiley & Sons, 2003
-
-
For selected examples, see:
- Zheng Y. Li X. Ren C. Zhang-Negrerie D. Du Y. Zhao K. J. Org. Chem. 2012;77:10353–10361. doi: 10.1021/jo302073e. - DOI - PubMed
- Cheung C. W. Buchwald S. L. J. Org. Chem. 2012;77:7526–7537. doi: 10.1021/jo301332s. - DOI - PMC - PubMed
- Senadi G. C. Hu W.-P. Hsiao J.-S. Vandavasi J. K. Chen C.-Y. Wang J.-J. Org. Lett. 2012;14:4478–4481. doi: 10.1021/ol301980g. - DOI - PubMed
- Hu Y. Yi R. Wang C. Xin X. Wu F. Wan B. J. Org. Chem. 2014;79:3052–3059. doi: 10.1021/jo5001719. - DOI - PubMed
- Weng Y. Lv W. Yu J. Ge B. Cheng G. Org. Lett. 2018;20:1853–1856. doi: 10.1021/acs.orglett.8b00376. - DOI - PubMed
- Newar U. D. Borra S. Maurya R. A. Org. Lett. 2022;24:4454–4458. doi: 10.1021/acs.orglett.2c01691. - DOI - PubMed
-
-
-
For selected examples, see:
- Cano I. Álvarez E. Nicasio M. C. Pérez P. J. J. Am. Chem. Soc. 2011;133:191–193. doi: 10.1021/ja109732s. - DOI - PubMed
- Li X. Huang L. Chen H. Wu W. Huang H. Jiang H. Chem. Sci. 2012;3:3463–3767. doi: 10.1039/C2SC21041J. - DOI
- Xu Z. Zhang C. Jiao N. Angew. Chem., Int. Ed. 2012;51:11367–11370. doi: 10.1002/anie.201206382. - DOI - PubMed
- Odabachian Y. Tong S. Wang Q. Wang M.-X. Zhu J. Angew. Chem., Int. Ed. 2013;52:10878–10882. doi: 10.1002/anie.201305506. - DOI - PubMed
- Di Mauro G. Maryasin B. Kaiser D. Shaaban S. González L. Maulide N. Org. Lett. 2017;19:3815–3818. doi: 10.1021/acs.orglett.7b01678. - DOI - PMC - PubMed
-
LinkOut - more resources
Full Text Sources

