Unveiling the roles of Sertoli cells lineage differentiation in reproductive development and disorders: a review
- PMID: 38699384
- PMCID: PMC11063913
- DOI: 10.3389/fendo.2024.1357594
Unveiling the roles of Sertoli cells lineage differentiation in reproductive development and disorders: a review
Abstract
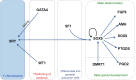
In mammals, gonadal somatic cell lineage differentiation determines the development of the bipotential gonad into either the ovary or testis. Sertoli cells, the only somatic cells in the spermatogenic tubules, support spermatogenesis during gonadal development. During embryonic Sertoli cell lineage differentiation, relevant genes, including WT1, GATA4, SRY, SOX9, AMH, PTGDS, SF1, and DMRT1, are expressed at specific times and in specific locations to ensure the correct differentiation of the embryo toward the male phenotype. The dysregulated development of Sertoli cells leads to gonadal malformations and male fertility disorders. Nevertheless, the molecular pathways underlying the embryonic origin of Sertoli cells remain elusive. By reviewing recent advances in research on embryonic Sertoli cell genesis and its key regulators, this review provides novel insights into sex determination in male mammals as well as the molecular mechanisms underlying the genealogical differentiation of Sertoli cells in the male reproductive ridge.
Keywords: Sertoli cell; embryonic cell development; mice; primordial germ cell; sex determination; spermatogenesis.
Copyright © 2024 Gao, Wang, Long, Yang, Jiang, Ding, Teng, Chen, Yuan and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




Similar articles
-
Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression.Development. 2017 Jan 1;144(1):44-53. doi: 10.1242/dev.144105. Epub 2016 Nov 25. Development. 2017. PMID: 27888191
-
Differentiation roadmap of embryonic Sertoli cells derived from mouse embryonic stem cells.Stem Cell Res Ther. 2019 Mar 8;10(1):81. doi: 10.1186/s13287-019-1180-6. Stem Cell Res Ther. 2019. PMID: 30850007 Free PMC article.
-
The Gonadal Supporting Cell Lineage and Mammalian Sex Determination: The Differentiation of Sertoli and Granulosa Cells.Results Probl Cell Differ. 2016;58:47-66. doi: 10.1007/978-3-319-31973-5_3. Results Probl Cell Differ. 2016. PMID: 27300175 Review.
-
Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2.Development. 2002 Oct;129(19):4627-34. doi: 10.1242/dev.129.19.4627. Development. 2002. PMID: 12223418
-
Testicular morphogenesis: comparison of in vivo and in vitro models to study male gonadal development.Ann N Y Acad Sci. 2007 Dec;1120:152-67. doi: 10.1196/annals.1411.015. Ann N Y Acad Sci. 2007. PMID: 18184913 Review.
Cited by
-
Testis-enriched Socs7 is not essential for spermatogenesis and male fertility in mice.Am J Transl Res. 2025 Mar 15;17(3):1780-1791. doi: 10.62347/VIVI6495. eCollection 2025. Am J Transl Res. 2025. PMID: 40226015 Free PMC article.
-
Unmasking the Epigenome: Insights into Testicular Cell Dynamics and Reproductive Function.Int J Mol Sci. 2025 Jul 28;26(15):7305. doi: 10.3390/ijms26157305. Int J Mol Sci. 2025. PMID: 40806437 Free PMC article. Review.
-
Physiological Functions of Urea Transporters.Subcell Biochem. 2025;118:105-125. doi: 10.1007/978-981-96-6898-4_6. Subcell Biochem. 2025. PMID: 40637979 Review.
-
Dynamic transcriptomic and regulatory networks underpinning the transition from fetal primordial germ cells to spermatogonia in mice.Cell Prolif. 2025 Feb;58(2):e13755. doi: 10.1111/cpr.13755. Epub 2024 Sep 27. Cell Prolif. 2025. PMID: 39329203 Free PMC article.
-
Exposure to polystyrene nanoplastics impairs sperm metabolism and pre-implantation embryo development in mice.Front Cell Dev Biol. 2025 Feb 28;13:1562331. doi: 10.3389/fcell.2025.1562331. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40092630 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

