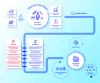
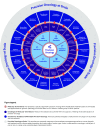
Challenges and solutions to system-wide use of precision oncology as the standard of care paradigm
- PMID: 38699518
- PMCID: PMC11062796
- DOI: 10.1017/pcm.2024.1
Challenges and solutions to system-wide use of precision oncology as the standard of care paradigm
Abstract
The personalised oncology paradigm remains challenging to deliver despite technological advances in genomics-based identification of actionable variants combined with the increasing focus of drug development on these specific targets. To ensure we continue to build concerted momentum to improve outcomes across all cancer types, financial, technological and operational barriers need to be addressed. For example, complete integration and certification of the 'molecular tumour board' into 'standard of care' ensures a unified clinical decision pathway that both counteracts fragmentation and is the cornerstone of evidence-based delivery inside and outside of a research setting. Generally, integrated delivery has been restricted to specific (common) cancer types either within major cancer centres or small regional networks. Here, we focus on solutions in real-world integration of genomics, pathology, surgery, oncological treatments, data from clinical source systems and analysis of whole-body imaging as digital data that can facilitate cost-effectiveness analysis, clinical trial recruitment, and outcome assessment. This urgent imperative for cancer also extends across the early diagnosis and adjuvant treatment interventions, individualised cancer vaccines, immune cell therapies, personalised synthetic lethal therapeutics and cancer screening and prevention. Oncology care systems worldwide require proactive step-changes in solutions that include inter-operative digital working that can solve patient centred challenges to ensure inclusive, quality, sustainable, fair and cost-effective adoption and efficient delivery. Here we highlight workforce, technical, clinical, regulatory and economic challenges that prevent the implementation of precision oncology at scale, and offer a systematic roadmap of integrated solutions for standard of care based on minimal essential digital tools. These include unified decision support tools, quality control, data flows within an ethical and legal data framework, training and certification, monitoring and feedback. Bridging the technical, operational, regulatory and economic gaps demands the joint actions from public and industry stakeholders across national and global boundaries.
Keywords: cost-effectiveness; health data; interoperability; patient centred; patient record; precision medicine; precision oncology; standard of care.
© The Author(s) 2024.
Conflict of interest statement
A.H., A.O., L.O., M.S.P. and N.L. are employed by Roche. S.A.V., A.T., K.W., C.C., T.N., H.S., K.A.V., H.B.-W., K.M.B., L.B., D.R.M. and J.D. have no conflict of interest to disclose related to this publication. A.B.H. has no conflicts of interest with the exception of holding a research grant award at the University of Oxford from Hofmann-La Roche, Cancer Research UK, Grenfell-Shaw charity, NIHR UK, Kennel Club Charitable Trust and EPA trust.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
-
Critical Care Network in the State of Qatar.Qatar Med J. 2019 Nov 7;2019(2):2. doi: 10.5339/qmj.2019.qccc.2. eCollection 2019. Qatar Med J. 2019. PMID: 31763205 Free PMC article.
-
Integrated, cross-sectoral psycho-oncology (isPO): a new form of care for newly diagnosed cancer patients in Germany.BMC Health Serv Res. 2022 Apr 22;22(1):543. doi: 10.1186/s12913-022-07782-0. BMC Health Serv Res. 2022. PMID: 35459202 Free PMC article.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
Cited by
-
Unveiling Pharmacogenomics Insights into Circular RNAs: Toward Precision Medicine in Cancer Therapy.Biomolecules. 2025 Apr 5;15(4):535. doi: 10.3390/biom15040535. Biomolecules. 2025. PMID: 40305280 Free PMC article. Review.
-
Implementing Personalized Cancer Medicine: Insights from a Qualitative Interview Study.J Pers Med. 2025 Apr 9;15(4):150. doi: 10.3390/jpm15040150. J Pers Med. 2025. PMID: 40278329 Free PMC article.
References
-
- Abernethy A, Adams L, Barrett M, Bechtel C, Brennan P, Butte A, Faulkner J, Fontaine E, Friedhoff S, Halamka J, Howell M, Johnson K, Long P, McGraw D, Miller R, Lee P, Perlin J, Rucker D, Sandy L, Savage L, Stump L, Tang P, Topol E, Tuckson R and Valdes K (2022) The promise of digital health: then, now, and the future. National Academy of Medicine Perspectives 2022, 1–24. 10.31478/202206e. - DOI - PMC - PubMed
-
- Baird A-M, Westphalen CB, Blum S, Nafria B, Knott T, Sargeant I, Harnik H, Brooke N, Wicki N and Wong-Rieger D (2023) How can we deliver on the promise of precision medicine in oncology and beyond? A practical roadmap for action. Health Science Reports 6(6), e1349. 10.1002/hsr2.1349. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources