High-dose pulsed hyaluronidase for managing nasal skin necrosis following hyaluronic acid treatment in nasolabial folds: A case report
- PMID: 38699685
- PMCID: PMC11063503
- DOI: 10.1016/j.jobcr.2024.04.006
High-dose pulsed hyaluronidase for managing nasal skin necrosis following hyaluronic acid treatment in nasolabial folds: A case report
Erratum in
-
Erratum regarding missing Ethical statements in previously published articles.J Oral Biol Craniofac Res. 2024 Sep-Oct;14(5):668. doi: 10.1016/j.jobcr.2024.09.006. Epub 2024 Sep 17. J Oral Biol Craniofac Res. 2024. PMID: 39318850 Free PMC article.
Abstract
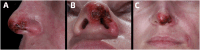
The growing popularity of soft tissue filler injections has brought attention to the associated risks, particularly vascular complications, and their treatments. This case report focuses on a 34-year-old female who developed nasal skin necrosis following hyaluronic acid (HA) filler injection for nasolabial fold (NLF) enhancement. Despite the careful procedure, complications emerged rapidly, emphasizing the critical importance of prompt diagnosis and intervention. A total of 10,000 turbidity reducing units (TRU) of hyaluronidase (HSE) were administered in a high-dose pulsed manner, alongside hyperbaric oxygen therapy. The patient experienced a gradual but significant improvement over 60 days. This case underscores the need for constant vigilance in aesthetic medicine and the potential consequences of even minute HA amounts, exceeding zero, in causing severe vascular events.
Keywords: Hyaluronic acid; Ischemia; Necrosis.
© 2024 The Authors. Published by Elsevier B.V. on behalf of Craniofacial Research Foundation.
Conflict of interest statement
The authors report no conflict of interests.
Figures



References
-
- Xiong M., Chen C., Sereda Y., Garibyan L., Avram M., Lee K.C. Retrospective analysis of the MAUDE database on dermal filler complications from 2014–2020. J Am Acad Dermatol. 2022;87(5):1158–1160. - PubMed
-
- Quezada-Gaón N., Wortsman X. Ultrasound-guided hyaluronidase injection in cosmetic complications. J Eur Acad Dermatol Venereol. 2016;30(10):e39–e40. - PubMed
-
- Cohen J.L., Biesman B.S., Dayan S.H., et al. Treatment of hyaluronic acid filler–induced impending necrosis with hyaluronidase: consensus recommendations. Aesthetic Surg J. 2015;35(7):844–849. - PubMed
-
- DeLorenzi C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthetic Surg J. 2017;37(7):814–825. - PubMed
LinkOut - more resources
Full Text Sources

