Advance Care Planning for Children With Rare Diseases: A Pilot RCT
- PMID: 38699801
- PMCID: PMC11153326
- DOI: 10.1542/peds.2023-064557
Advance Care Planning for Children With Rare Diseases: A Pilot RCT
Abstract
Background and objective: Pediatric rare diseases are often life-limiting conditions and/or require constant caregiving. Investigators assessed the initial efficacy of the FAmily CEntered (FACE) pediatric advance care planning (pACP), FACE-Rare, intervention on families' quality of life.
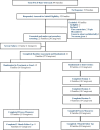
Methods: A pilot-phase, single-blinded, intent-to-treat, randomized controlled clinical trial enrolled families from 1 pediatric quaternary hospital between 2021 and 2023. Intervention families received 3 weekly 60-minute (FACE-Rare pACP) sessions: (1) Carer Support Needs Assessment Tool or Action Plan, (2) Carer Support Needs Assessment Tol Action Plan Review, and (3) Pediatric Next Steps: Respecting Choices pACP. Controls received treatment as usual (TAU). Outcome measures were Beck Anxiety Inventory, Family Appraisal of Caregiving, Functional Assessment of Chronic Illness Therapy (FACIT)-Spirituality, and health care utilization. Generalized mixed effect models with γ response assessed the intervention effect at 3-month follow-up.
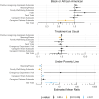
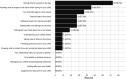
Results: Children (n = 21) were aged 1 to 10 years, 48% male, 24% Black; and 100% technology dependent. Primary family caregivers (n = 21) were aged 30 to 43 years, 19% male, 19% Black; and 27% household income below the Federal poverty level. Dyads underwent 1:1 randomization: 9 to FACE-Rare and 12 to TAU. TAU caregivers reported statistically lower meaning and peace than FACE-Rare caregivers (0.9, P = .03, confidence interval [CI]: 0.75-0.99). Black caregivers reported significantly less caregiver distress (0.7, P = .04, CI: 0.47-0.98) than non-Black caregivers. Poor families reported more anxiety (3.5, P = .002, CI: 1.62-7.94), more caregiver strain (1.2, P = .006, CI: 1.07-1.42); and less family well-being (0.8, P = .02, CI: 0.64-0.95).
Conclusions: FACE®-Rare was feasible, acceptable, safe, and demonstrated initial efficacy, providing greater feelings of meaning and peace to caregivers. Poverty impacted well-being. A multisite trial is needed to determine generalizability.
Copyright © 2024 by the American Academy of Pediatrics.
Conflict of interest statement
Figures
Similar articles
-
Family caregivers of children and adolescents with rare diseases: a novel palliative care intervention.BMJ Support Palliat Care. 2022 Nov;12(e5):e705-e714. doi: 10.1136/bmjspcare-2019-001766. Epub 2019 Jul 25. BMJ Support Palliat Care. 2022. PMID: 31345846 Free PMC article.
-
Effect of the Family-Centered Advance Care Planning for Teens with Cancer Intervention on Sustainability of Congruence About End-of-Life Treatment Preferences: A Randomized Clinical Trial.JAMA Netw Open. 2022 Jul 1;5(7):e2220696. doi: 10.1001/jamanetworkopen.2022.20696. JAMA Netw Open. 2022. PMID: 35819787 Free PMC article. Clinical Trial.
-
Pediatric Advance Care Planning and Families' Positive Caregiving Appraisals: An RCT.Pediatrics. 2021 Jun;147(6):e2020029330. doi: 10.1542/peds.2020-029330. Epub 2021 May 6. Pediatrics. 2021. PMID: 33958436 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Telephone interventions, delivered by healthcare professionals, for providing education and psychosocial support for informal caregivers of adults with diagnosed illnesses.Cochrane Database Syst Rev. 2019 May 14;5(5):CD012533. doi: 10.1002/14651858.CD012533.pub2. Cochrane Database Syst Rev. 2019. PMID: 31087641 Free PMC article.
Cited by
-
Evaluation of the Health-Related Quality of Life and Mental Health of Parents With Children and Adolescents With a Rare Disease Based on the Results of a Randomized Controlled Trial to Investigate a Family-Based Intervention and an Online Intervention for Affected Families (CARE-FAM-NET).Fam Process. 2025 Jun;64(2):e70041. doi: 10.1111/famp.70041. Fam Process. 2025. PMID: 40375458 Free PMC article. Clinical Trial.
References
-
- National Human Genome Research Institute. Rare diseases FAQ. Available at: https://www.genome.gov/FAQ/Rare-Diseases. Accessed September 20, 2023
-
- The Hastings Center. Family caregiving 2016. Available at: www.thehastingscenter.org/briefingbook/family-caregiving/. Accessed September 20, 2023
-
- Global Genes. RARE diseases: facts and statistics 2016. Available at: https://globalgenes.org/rare-diseases-facts-statistics/. Accessed September 20, 2023
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical