Monocyte-Derived Macrophages Aggravate Cardiac Dysfunction After Ischemic Stroke in Mice
- PMID: 38700011
- PMCID: PMC11179859
- DOI: 10.1161/JAHA.123.034731
Monocyte-Derived Macrophages Aggravate Cardiac Dysfunction After Ischemic Stroke in Mice
Abstract
Background: Cardiac damage induced by ischemic stroke, such as arrhythmia, cardiac dysfunction, and even cardiac arrest, is referred to as cerebral-cardiac syndrome (CCS). Cardiac macrophages are reported to be closely associated with stroke-induced cardiac damage. However, the role of macrophage subsets in CCS is still unclear due to their heterogeneity. Sympathetic nerves play a significant role in regulating macrophages in cardiovascular disease. However, the role of macrophage subsets and sympathetic nerves in CCS is still unclear.
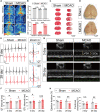
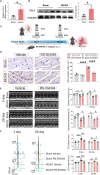
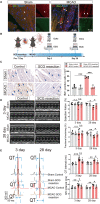
Methods and results: In this study, a middle cerebral artery occlusion mouse model was used to simulate ischemic stroke. ECG and echocardiography were used to assess cardiac function. We used Cx3cr1GFPCcr2RFP mice and NLRP3-deficient mice in combination with Smart-seq2 RNA sequencing to confirm the role of macrophage subsets in CCS. We demonstrated that ischemic stroke-induced cardiac damage is characterized by severe cardiac dysfunction and robust infiltration of monocyte-derived macrophages into the heart. Subsequently, we identified that cardiac monocyte-derived macrophages displayed a proinflammatory profile. We also observed that cardiac dysfunction was rescued in ischemic stroke mice by blocking macrophage infiltration using a CCR2 antagonist and NLRP3-deficient mice. In addition, a cardiac sympathetic nerve retrograde tracer and a sympathectomy method were used to explore the relationship between sympathetic nerves and cardiac macrophages. We found that cardiac sympathetic nerves are significantly activated after ischemic stroke, which contributes to the infiltration of monocyte-derived macrophages and subsequent cardiac dysfunction.
Conclusions: Our findings suggest a potential pathogenesis of CCS involving the cardiac sympathetic nerve-monocyte-derived macrophage axis.
Keywords: NLRP3 inflammasome; cardiac dysfunction; ischemic stroke; monocyte‐derived macrophage; sympathetic nerve.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

