Etrasimod for the Treatment of Ulcerative Colitis: Analysis of Infection Events from the ELEVATE UC Clinical Programme
- PMID: 38700040
- PMCID: PMC11479712
- DOI: 10.1093/ecco-jcc/jjae060
Etrasimod for the Treatment of Ulcerative Colitis: Analysis of Infection Events from the ELEVATE UC Clinical Programme
Abstract
Background and aims: Infections are a safety concern in patients with ulcerative colitis [UC]. Etrasimod is an oral, once daily [QD], selective sphingosine 1-phosphate [S1P]1,4,5 receptor modulator for the treatment of moderately to severely active UC. It leads to selective and reversible lymphocyte sequestration and partial peripheral lymphocyte count decrease. We report infection events from the phase 3 ELEVATE programme.
Methods: Proportions, incidence rates [IRs; per 100 patient-years], and descriptive analyses of all serious, severe, herpes zoster and opportunistic infections are reported in the Pivotal UC cohort [ELEVATE UC 52 and ELEVATE UC 12]. Cox regression models evaluated potential baseline risk factors.
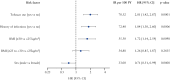
Results: In this analysis [n = 787], proportions [IRs] of all infection events were similar for patients receiving etrasimod 2 mg QD (18.8% [41.1]) or placebo (17.7% [49.0]). Serious infections occurred in three [0.6%] and five [1.9%] patients receiving etrasimod and placebo, respectively. Two herpes zoster events were reported in each group [etrasimod: 0.4%; placebo: 0.8%], all localised and non-serious. One opportunistic infection event was reported in each group. No patient with an absolute lymphocyte count [ALC] < 0.2 × 109/L reported serious/severe or opportunistic infections; no baseline risk factors were identified for such events. No deaths occurred.
Conclusions: Patients receiving etrasimod demonstrated no increased risk of infection. The incidence of serious infections and herpes zoster was similar in each group. Among patients receiving etrasimod, no association between ALC < 0.5 × 109/L and infection events was observed. Longer-term follow-up will further characterise the etrasimod safety profile. Clinicaltrials.gov: NCT03945188; NCT03996369.
Keywords: Etrasimod; infections; ulcerative colitis.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
MR reports grants and research support from AbbVie, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, Pfizer, Takeda, and UCB; consultancy fees from AbbVie, ALFASIGMA, S.p.A., Allergan, Amgen, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, Lilly, Miraca Labs, Pfizer, Prometheus, Salix, Seres, Takeda, TARGET Pharma Solutions, and UCB; and holds stock in Wolters Kluwer Health as an Author/Editor of UpToDate. BS reports research support from Pfizer; lecture fees from AbbVie, CED Service GmbH, Chiesi, Falk, Ferring, Gilead, Janssen, Lilly, Materia Prima, Pfizer, and Takeda; and consultancy fees from AbbVie, Abivax, Arena Pharma, Bristol Myers Squibb, Boehringer, CED Service GmbH, Celgene, CT-Scout, Endpoint Health, Falk, Forga Software, Galapagos, Gilead, Janssen, Lilly, Materia Prima, Pfizer, Pharma Insight, PredictImmune, PsiCro, and Takeda. AJY reports consultancy fees from Arena, Bristol Myers Squibb, Pfizer, and Takeda; and lecture/speaker fees from Bristol Myers Squibb. FS reports consultancy/speaker fees from AbbVie, Amgen, Celltrion, Eurofarma, Ferring, Janssen, Pfizer, Sandoz, and Takeda. KBG reports research support from Celltrion, Galapagos, and Pfizer; and speakers’ honoraria and/or consultancy fees from AbbVie, Celltrion, Ferring Pharmaceuticals, Immunic Therapeutics, Janssen, Novartis, Pfizer Inc, Samsung Bioepis, Takeda, and Tillotts. MG is an employee and shareholder of Pfizer AG. AB is an employee of Pfizer Healthcare India Pvt; and shareholder of Pfizer Inc. JW, JG, CC, DB, and IM are employees and shareholders of Pfizer Inc. AM is an employee of Pfizer Ltd, and a shareholder of Pfizer. KL is an employee of Pfizer AG, and a shareholder of Pfizer. MTA reports consulting fees from AbbVie, Bristol Myers Squibb, Celsius, Eli Lilly, Gilead, Janssen, Janssen Biotech, Pfizer, Prometheus, Prometheus Bioscience, Takeda, and UCB Biopharma.
Figures


References
-
- Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F.. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis 2014;20:2198–206. - PubMed
-
- Kucharzik T, Ellul P, Greuter T, et al.. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis 2021;15:879–913. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

