Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients
- PMID: 38700045
- PMCID: PMC11066972
- DOI: 10.1002/14651858.CD003774.pub5
Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients
Abstract
Background: The risk of cytomegalovirus (CMV) infection in solid organ transplant recipients has resulted in the frequent use of prophylaxis to prevent the clinical syndrome associated with CMV infection. This is an update of a review first published in 2005 and updated in 2008 and 2013.
Objectives: To determine the benefits and harms of antiviral medications to prevent CMV disease and all-cause death in solid organ transplant recipients.
Search methods: We contacted the information specialist and searched the Cochrane Kidney and Transplant Register of Studies up to 5 February 2024 using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs comparing antiviral medications with placebo or no treatment, comparing different antiviral medications or different regimens of the same antiviral medications for CMV prophylaxis in recipients of any solid organ transplant. Studies examining pre-emptive therapy for CMV infection are studied in a separate review and were excluded from this review.
Data collection and analysis: Two authors independently assessed study eligibility, risk of bias and extracted data. Summary estimates of effect were obtained using a random-effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results: This 2024 update found four new studies, bringing the total number of included studies to 41 (5054 participants). The risk of bias was high or unclear across most studies, with a low risk of bias for sequence generation (12), allocation concealment (12), blinding (11) and selective outcome reporting (9) in fewer studies. There is high-certainty evidence that prophylaxis with aciclovir, ganciclovir or valaciclovir compared with placebo or no treatment is more effective in preventing CMV disease (19 studies: RR 0.42, 95% CI 0.34 to 0.52), all-cause death (17 studies: RR 0.63, 95% CI 0.43 to 0.92), and CMV infection (17 studies: RR 0.61, 95% CI 0.48 to 0.77). There is moderate-certainty evidence that prophylaxis probably reduces death from CMV disease (7 studies: RR 0.26, 95% CI 0.08 to 0.78). Prophylaxis reduces the risk of herpes simplex and herpes zoster disease, bacterial and protozoal infections but probably makes little to no difference to fungal infection, acute rejection or graft loss. No apparent differences in adverse events with aciclovir, ganciclovir or valaciclovir compared with placebo or no treatment were found. There is high certainty evidence that ganciclovir, when compared with aciclovir, is more effective in preventing CMV disease (7 studies: RR 0.37, 95% CI 0.23 to 0.60). There may be little to no difference in any outcome between valganciclovir and IV ganciclovir compared with oral ganciclovir (low certainty evidence). The efficacy and adverse effects of valganciclovir or ganciclovir were probably no different to valaciclovir in three studies (moderate certainty evidence). There is moderate certainty evidence that extended duration prophylaxis probably reduces the risk of CMV disease compared with three months of therapy (2 studies: RR 0.20, 95% CI 0.12 to 0.35), with probably little to no difference in rates of adverse events. Low certainty evidence suggests that 450 mg/day valganciclovir compared with 900 mg/day valganciclovir results in little to no difference in all-cause death, CMV infection, acute rejection, and graft loss (no information on adverse events). Maribavir may increase CMV infection compared with ganciclovir (1 study: RR 1.34, 95% CI: 1.10 to 1.65; moderate certainty evidence); however, little to no difference between the two treatments were found for CMV disease, all-cause death, acute rejection, and adverse events at six months (low certainty evidence).
Authors' conclusions: Prophylaxis with antiviral medications reduces CMV disease and CMV-associated death, compared with placebo or no treatment, in solid organ transplant recipients. These data support the continued routine use of antiviral prophylaxis in CMV-positive recipients and CMV-negative recipients of CMV-positive organ transplants.
Trial registration: ClinicalTrials.gov NCT00294515 NCT00227370 NCT00497796 NCT03699254 NCT00947141 NCT01753167 NCT02550639 NCT00374686 NCT00373165 NCT00566072 NCT01446445 NCT01611974 NCT00966836 NCT01552369 NCT00431353 NCT00372229 NCT03443869 NCT01329185 NCT04225923.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Robin Vernooij: no relevant interests were disclosed
Mini Michael: no relevant interests were disclosed
Maleeka Ladhani: no relevant interests were disclosed
Angela Webster: no relevant interests were disclosed
Giovanni Strippoli: no relevant interests were disclosed
Jonathan Craig: no relevant interests were disclosed
Elisabeth Hodson: no relevant interests were disclosed
Figures





















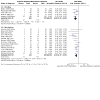


















































Update of
-
Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD003774. doi: 10.1002/14651858.CD003774.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2024 Apr 10;4:CD004667. doi: 10.1002/14651858.CD004667.pub6. Update in: Cochrane Database Syst Rev. 2024 May 3;5:CD003774. doi: 10.1002/14651858.CD003774.pub5. PMID: 23450543 Updated.
References
References to studies included in this review
2VAL 2010 Kidney {published data only}
-
- Kacer M, Kielberger L, Bouda M, Reischig T. Pharmacoeconomic impact of valganciclovir versus valacyclovir for cytomegalovirus prophylaxis in renal transplant recipients: a randomized controlled trial [abstract no: D2366]. Transplantation 2014;98(Suppl 1):763. [EMBASE: 71546145]
-
- Kacer M, Kielberger L, Bouda M, Reischig T. Valganciclovir versus valacyclovir prophylaxis for prevention of cytomegalovirus: an economic perspective. Transplant Infectious Disease 2015;17(3):334-41. [MEDLINE: ] - PubMed
-
- Reischig T, Jindra P, Hes O, Lysak D, Bouda M. Randomized comparison of valganciclovir and valacyclovir for cytomegalovirus prophylaxis [abstract no: O026]. Transplant International 2013;26(Suppl 2):29. [EMBASE: 71359052]
-
- Reischig T, Jindra P, Hes O, Lysak D, Bouda M. The 2VAL Study: Randomized comparison of valganciclovir vs valacyclovir for cytomegalovirus prophylaxis after renal transplantation [abstract no: LB19]. American Journal of Transplantation 2012;12(Suppl 3):361. [EMBASE: 70747097]
-
- Reischig T, Jindra P, Hes O, Lysak D, Bouda M. The 2VAL study: Randomized comparison of valganciclovir vs. valacyclovir for cytomegalovirus prophylaxis after renal transplantation [abstract no: 1342]. Transplantation 2012;94(Suppl 10S):541. [EMBASE: 71250846]
Ahsan 1997 Kidney {published data only}
-
- Ahsan N, Holman MJ, Dhilon S, Ream L, Yang HC. Oral ganciclovir (CytoveneR) effectively prevents cytomegalovirus (CMV) infection in renal transplant patients [abstract no: A3406]. Journal of the American Society of Nephrology 1996;7(9):1929. [CENTRAL: CN-00583884]
-
- Ahsan N, Holman MJ, Yang HC. Efficacy of oral ganciclovir in prevention of cytomegalovirus infection in post-kidney transplant patients. Clinical Transplantation 1997;11(6):633-9. [MEDLINE: ] - PubMed
-
- Ahsan N, Holman MJ, Yang HC. Oral ganciclovir is effective in preventing CMV infection in renal transplant recipients [abstract]. Nephrology 1997;3(Suppl 1):S70. [CENTRAL: CN-00460256]
Ali Ibrahim 2020 Kidney {published data only}
-
- Ali Ibrahim M, Mansour AR, Mohamed El-Okely A, Mohamed Goda T, Hosny Zahran M, Refaie AF, et al. Three-year follow up of cytomegalovirus prophylaxis regimens in renal transplant recipients as an economy-saving strategy: a comparative study [abstract no: SAT-321}. Kidney International Reports 2020;5(3 Suppl):S135. [EMBASE: 2005255599]
Badley 1997 Liver {published data only}
-
- Badley AD, Seaberg EC, Porayko MK, Wiesner RH, Keating MR, Wilhelm MP, et al. Prophylaxis of cytomegalovirus infection in liver transplantation: a randomized trial comparing a combination of ganciclovir and acyclovir to acyclovir. NIDDK Liver Transplantation Database. Transplantation 1997;64(1):66-73. [MEDLINE: ] - PubMed
-
- Kim WR, Badley AD, Wiesner RH, Porayko MK, Seaberg EC, Keating MR, et al. The economic impact of cytomegalovirus infection after liver transplantation. Transplantation 2000;69(3):357-61. [MEDLINE: ] - PubMed
-
- Paya CV, Marin E, Keating M, Dickson R, Porayko M, Wiesner R. Solid organ transplantation: results and implications of acyclovir use in liver transplants. Journal of Medical Virology 1993;Suppl 1:123-7. [MEDLINE: ] - PubMed
Balfour 1989 Kidney {published data only}
-
- Balfour HH Jr, Bean B, Mitchell CD, Sachs GW, Boen JR, Edelman CK. Acyclovir in immunocompromised patients with cytomegalovirus disease. A controlled trial at one institution. American Journal of Medicine 1982;73(1A):241-8. [MEDLINE: ] - PubMed
-
- Balfour HH Jr, Chace BA, Stapleton JT, Simmons RL, Fryd DS. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. New England Journal of Medicine 1989;320(21):1381-7. [MEDLINE: ] - PubMed
-
- Balfour HH Jr, Fletcher CV, Dunn D. Prevention of cytomegalovirus disease with oral acyclovir. Transplantation Proceedings 1991;23(2 Suppl 1):17-9. [MEDLINE: ] - PubMed
-
- Balfour HH Jr. Prevention of cytomegalovirus disease in renal allograft recipients. Scandinavian Journal of Infectious Diseases Supplement 1991;80:88-93. [MEDLINE: ] - PubMed
Barkholt 1999 Liver {published data only}
-
- Barkholt L, Lewensohn-Fuchs I, Ericzon BG, Tyden G, Andersson J. High-dose acyclovir prophylaxis reduces cytomegalovirus disease in liver transplant patients. Transplant Infectious Disease 1999;1(2):89-97. [MEDLINE: ] - PubMed
Brennan 1997 Kidney {published data only}
-
- Brennan DC, Garlock KA, Singer GG, Schnitzler MA, Lippmann BJ, Buller RS, et al. Prophylactic oral ganciclovir compared with deferred therapy for control of cytomegalovirus in renal transplant recipients. Transplantation 1997;64(12):1843-6. [MEDLINE: ] - PubMed
-
- Brennan DC, Garlock KA, Singer GG, Schnizler MA, Lippmann BJ, Buller RS, et al. Prophylactic oral ganciclovir prevents cytomegalovirus infection and disease in renal transplant recipients [abstract no: 11]. In: 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10-14; Chicago (ILL). 1997:87. [CENTRAL: CN-00509103]
-
- Brennan DC, Singer GG, Garlock KA, Schnitzler MA, Storch GA. Prophylactic oral ganciclovir prevents cytomegalovirus disease in renal transplant recipients [abstract no: P490]. Nephrology 1997;3(Suppl 1):S197. [CENTRAL: CN-00460455]
-
- Brennan DC, Storch GA, Singer GG, Lee L, Rueda J, Schnitzler MA. The prevalence of human herpesvirus-7 in renal transplant recipients is unaffected by oral or intravenous ganciclovir. Journal of Infectious Diseases 2000;181(5):1557-61. [MEDLINE: ] - PubMed
-
- Singer GG, Storch GA, Burton KG, Lippmann BJ, Buller RS, Gaudreault-Keener M, et al. Prophylactic oral ganciclovir prevents cytomegalovirus disease in high-risk renal transplant recipients [abstract no: A3469]. Journal of the American Society of Nephrology 1996;7(9):1941. [CENTRAL: CN-00766068]
Cohen 1993 Liver {published data only}
-
- Cohen AT, O'Grady JG, Sutherland S, Sallie R, Tan KC, Williams R. Controlled trial of prophylactic versus therapeutic use of ganciclovir after liver transplantation in adults. Journal of Medical Virology 1993;40(1):5-9. [MEDLINE: ] - PubMed
Conti 1995 Kidney {published data only}
-
- Conti DJ, Freed BM, Singh TP, Gallichio M, Gruber SA, Lempert N. Preemptive ganciclovir therapy in cytomegalovirus-seropositive renal transplants recipients. Archives of Surgery 1995;130(11):1217-22. [MEDLINE: ] - PubMed
Duncan 1993 Lung {published data only}
-
- Duncan SR, Grgurich WF, Iacono AT, Burckart GJ, Yousem SA, Paradis IL, et al. A comparison of ganciclovir and acyclovir to prevent cytomegalovirus after lung transplantation. American Journal of Respiratory & Critical Care Medicine 1994;150(1):146-52. [MEDLINE: ] - PubMed
Egan 2002 Heart {published data only}
-
- Egan JJ, Carroll KB, Yonan N, Woodcock A, Crisp A. Valacyclovir prevention of cytomegalovirus reactivation after heart transplantation: a randomized trial. Journal of Heart & Lung Transplantation 2002;21(4):460-6. [MEDLINE: ] - PubMed
Flechner 1998 Kidney {published data only}
-
- Flechner SM, Avery RK, Fisher R, Mastroianni BA, Papajcik DA, O'Malley KJ, et al. A randomized prospective controlled trial of oral acyclovir versus oral ganciclovir for cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Transplantation 1998;66(12):1682-8. [MEDLINE: ] - PubMed
-
- Flechner SM, O'Malley K, Fisher R, Mastroianni B, Papajcik D, Avery R, et al. A randomized prospective trial of oral acyclovir vs oral ganciclovir for CMV prophylaxis in high risk kidney transplant recipients [abstract no: 739]. Transplantation 1998;65(12):S187. [CENTRAL: CN-00644223] - PubMed
Gane 1997 Liver {published data only}
-
- Gane E, Saliba F, Valdecasas GJ, O'Grady J, Pescovitz MD, Lyman S, et al. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [Erratum in: Lancet 1998 Feb 7;351(9100):454]. Lancet 1997;350(9093):1729-33. [MEDLINE: ] - PubMed
-
- Pescovitz MD, Pruett TL, Brook B, McGory R, Jung D. Pharmacokinetics of oral ganciclovir in liver transplant recipients [abstract]. In: 15th Annual Meeting. American Society of Transplant Physicians (ASTP); 1996 May 10-14; Chicago (ILL). 1996. [CENTRAL: CN-01657982]
-
- Saliba F, Bismuth H, Gane E, Valdecasas G, O'Grady J, Behrend M, et al. A randomized double blind versus placebo multicenter study of efficacy and tolerance of oral ganciclovir in the prevention of cytomegalovirus disease in hepatic transplanted patients. Gastroenterologie Clinique et Biologique 1997;21(2 bis):A157. [CENTRAL: CN-00583127]
Gavalda 1997 Liver {published data only}
-
- Gavalda J, Otero J, Murio E, Vargas V, Rossello J, Calico I, et al. Two grams daily of oral acyclovir reduces the incidence of cytomegalovirus disease in CMV-seropositive liver transplant recipients. Transplant International 1997;10(6):462-5. [MEDLINE: ] - PubMed
Green 1997 Liver {published data only}
-
- Green M, Kaufmann M, Wilson J, Reyes J. Comparison of intravenous ganciclovir followed by oral acyclovir with intravenous ganciclovir alone for prevention of cytomegalovirus and Epstein-Barr virus disease after liver transplantation in children. Clinical Infectious Diseases 1997;25(6):1344-9. [MEDLINE: ] - PubMed
-
- Green M, Reyes J, Nour B, Beatty D, Kaufman M, Wilson J, et al. Randomized trial of ganciclovir followed by high-dose oral acyclovir vs ganciclovir alone in the prevention of cytomegalovirus disease in pediatric liver transplant recipients: preliminary analysis. Transplantation Proceedings 1994;26(1):173-4. [MEDLINE: ] - PubMed
Halim 2016 Kidney {published data only}
-
- Abdel-Halim M, Al-Otaibi T, Gheith O, Adel H, Mosaad A, Abo-Atya H, et al. Efficacy and safety of low dose versus full dose valganciclovir for prevention of cytomegalovirus disease in kidney transplant recipients [abstract no: BO71]. Transplant International 2015;28(Suppl 4):155. [EMBASE: 72111670]
-
- Gheith O, Al-Otaibi T, Halim A, Adel H, Mosaad A, Atteya H, et al. Successful cost effective prevention of cytomegalovirus disease in kidney transplant [abstract no: O02]. Transplant International 2015;28(Suppl 4):1. [EMBASE: 72111247]
-
- Gheith O, Al-Otaibi T, Halim MA, Mansour H, Attia H, Elsayed Z, et al. Successful cost effective prevention of cytomegalovirus disease in kidney transplant recipients using low dose valganciclovir [abstract no: MP689]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i568. [EMBASE: 72327538] - PubMed
-
- Gheith O, Halim MA, Al-Otaibi T, Mansour H, Mosaad A, Atteya HA, et al. Successful cost-effective prevention of cytomegalovirus disease in kidney transplant recipients using low-dose valganciclovir. Experimental & Clinical Transplantation 2017 Feb;15(Suppl 1):156-63. [MEDLINE: ] - PubMed
-
- Gheith O, Halim MA, Alotaibi T, Nair P, Said T, Nampoory N. Low dose valganciclovir prophylaxis among renal transplants: cost benefit analysis [abstract no: SP774]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii410. [EMBASE: 617291198]
Hertz 1998 Heart/lung {published data only}
-
- Hertz MI, Jordan C, Savik SK, Fox JM, Park S, Bolman RM 3rd, et al. Randomized trial of daily versus three-times-weekly prophylactic ganciclovir after lung and heart-lung transplantation. Journal of Heart & Lung Transplantation 1998;17(9):913-20. [MEDLINE: ] - PubMed
Hibberd 1995 Kidney {published data only}
-
- Hibberd PL, Tolkoff-Rubin NE, Conti D, Stuart F, Thistlethwaite JR, Neylan JF, et al. Preemptive ganciclovir therapy to prevent cytomegalovirus disease, in cytomegalovirus antibody-positive renal transplant recipients. A randomized controlled trial. Annals of Internal Medicine 1995;123(1):18-26. [MEDLINE: ] - PubMed
IMPACT 2010 Kidney {published data only}
-
- Blumberg E, Hauser I, Gahlemann CG, Berenson KL, Jardine A, Humar A. Cost-effectiveness model to evaluate 200-day vs 100-day valganciclovir (Valcyte®) prophylaxis to reduce CMV disease incidence post-transplant [abstract no: 290]. American Journal of Transplantation 2010;10(Suppl 4):126. [EMBASE: 70463651]
-
- Boivin G, Goyette N, Farhan M, Ives J, Elston R. Incidence of cytomegalovirus UL97 and UL54 amino acid substitutions detected after 100 or 200 days of valganciclovir prophylaxis. Journal of Clinical Virology 2012;53(3):208-13. [MEDLINE: ] - PubMed
-
- Chou S, Marousek G, Boivin G, Goyette N, Farhan M, Ives JA, et al. Recombinant phenotyping of cytomegalovirus sequence variants detected after 200 or 100 days of valganciclovir prophylaxis. Transplantation 2010;90(12):1409-13. [MEDLINE: ] - PubMed
-
- Chou S, Marousek G, Wong V, Boivin G, Goyette N, Farhan M, et al. The IMPACT study: Phenotypic analysis of previously uncharacterized cytomegalovirus UL54 and UL97 amino acid substitutions detected in virus from patients receiving 200 or 100 days of valganciclovir (Valcyte) prophylaxis [abstract no: 1099]. Transplantation 2010;90(Suppl 1):24. [EMBASE: 71531149]
-
- Elston R, Bovin G, Goyette N, Voulgari A, Ives J, Farhan M. The IMPACT Study: genotypic analysis of cytomegalovirus UL54 and UL97 genes derived from patients receiving 100 or 200 days of valganciclovir (Valcyte®) prophylaxis [abstract no: 580]. American Journal of Transplantation 2010;10(Suppl 4):208. [EMBASE: 70463941]
Kletzmayr 1996 Kidney {published data only}
-
- Kletzmayr J, Kotzmann H, Popow-Kraupp T, Kovarik J, Klauser R. Impact of high-dose oral acyclovir prophylaxis on cytomegalovirus (CMV) disease in CMV high-risk renal transplant recipients. Journal of the American Society of Nephrology 1996;7(2):325-30. [MEDLINE: ] - PubMed
-
- Kletzmayr J, Kotzmann H, Popow-Kraupp T, Kovarik J, Klauser R. Oral acyclovir in prevention of CMV disease in high-risk renal transplant recipients [abstract]. In: ISN XIII International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:375. [CENTRAL: CN-00509282]
Leray 1995 Kidney {published data only}
-
- Leray H, Mourad G, Chong G, Segondy M, Mion C. Prophylactic treatment of cytomegalovirus primary infection with ganciclovir in renal transplant recipients. Transplantation Proceedings 1995;27(4):2448. [MEDLINE: ] - PubMed
Lowance 1999 Kidney {published data only}
-
- Legendre CM, Norman DJ, Keating MR, Maclaine GD, Grant DM. Valaciclovir prophylaxis of cytomegalovirus infection and disease in renal transplantation: an economic evaluation. Transplantation 2000;70(10):1463-8. [MEDLINE: ] - PubMed
-
- Lowance D, Legendre C, Neumayer H, Norman D, Coggon G, Lee I, et al. Valaciclovir reduces the incidence of cytomegalovirus disease and acute graft rejection in CMV-seronegative recipients of a seropositive cadaveric renal allograft [abstract no: 61]. Transplantation 1998;65(12):S18. [CENTRAL: CN-00776893]
-
- Lowance D, Neumayer HH, Legendre CM, Squifflet JP, Kovarik J, Brennan PJ, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. New England Journal of Medicine 1998;340(19):1462-70. [MEDLINE: ] - PubMed
-
- Squifflet J, Mendez R. Valaciclovir reduces the incidence of cytomegalovirus disease in CMV-seropositive renal allograft recipients [abstract]. In: 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10-14; Chicago (ILL). 1997:87. [CENTRAL: CN-00509489]
Macdonald 1995 Heart {published data only}
-
- Macdonald PS, Keogh AM, Marshman D, Richens D, Harvison A, Kaan AM, et al. A double-blind placebo-controlled trial of low-dose ganciclovir to prevent cytomegalovirus disease after heart transplantation. Journal of Heart & Lung Transplantation 1995;14(1 Pt 1):32-8. [MEDLINE: ] - PubMed
Martin 1994 Liver {published data only}
-
- Martin M, Manez R, Linden P, Estores D, Torre-Cisneros J, Kusne S, et al. A prospective randomized trial comparing sequential ganciclovir-high dose acyclovir to high dose acyclovir for prevention of cytomegalovirus disease in adult liver transplant recipients. Transplantation 1994;58(7):779-85. [MEDLINE: ] - PMC - PubMed
Merigan 1992 Heart {published data only}
-
- Merigan TC, Renlund DG, Keay S, Bristow MR, Starnes V, O'Connell JB, et al. A controlled trial of ganciclovir to prevent cytomegalovirus disease after heart transplantation. New England Journal of Medicine 1992;326(18):1182-6. [MEDLINE: ] - PubMed
-
- Valantine HA, Gao SZ, Menon SG, Renlund DG, Hunt SA, Oyer P, et al. Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis: a post hoc analysis of a randomized, placebo-controlled study. Circulation 1999;100(1):61-6. [MEDLINE: ] - PubMed
-
- Wagner JA, Ross H, Hunt S, Gamberg P, Valantine H, Merigan T, et al. Prophylactic ganciclovir treatment reduces cytomegalovirus and fungal infections in orthotopic heart transplant recipients [abstract]. In: 14th Annual Meeting. American Society of Transplant Physicians (ASTP); 1995 May 14-17; Chicago (ILL). 1995.
-
- Wagner JA, Ross H, Hunt S, Gamberg P, Valantine H, Merigan TC, et al. Prophylactic ganciclovir treatment reduces fungal as well as cytomegalovirus infections after heart transplantation. Transplantation 1995;60(12):1473-7. [MEDLINE: ] - PubMed
Nafar 2005 Kidney {published data only}
-
- Nafar M, Pezeshki ML, Farrokhi F, Einollahi B, Pour-Reza-Gholi F, Firouzan A, et al. A randomized prospective trial of oral versus intravenous ganciclovir for prophylaxis of cytomegalovirus infection and disease in high-risk kidney recipients. Transplantation Proceedings 2005;37(7):3053-5. [MEDLINE: ] - PubMed
Nakazato 1993 Liver {published data only}
-
- Nakazato PZ, Burns W, Moore P, Garcia-Kennedy R, Cox K, Esquivel C. Viral prophylaxis in hepatic transplantation: Preliminary report of a randomized trial of acyclovir and gancyclovir. Transplantation Proceedings 1993;25(2):1935-7. [MEDLINE: ] - PubMed
Palmer 2010 Lung {published data only}
-
- Finlen Copeland CA, Davis WA, Snyder LD, Banks M, Avery R, Davis RD, et al. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. Journal of Heart & Lung Transplantation 2011;30(9):990-6. [MEDLINE: ] - PubMed
-
- Finlen Copeland CA, Davis WA, Snyder LD, Banks M, Avery R, Palmer SM. Reduced lifetime incidence of cytomegalovirus with extended prophylaxis: Long-term follow up from a randomized controlled trial [abstract no: 104]. Journal of Heart & Lung Transplantation 2011;30(4 Suppl 1):S42. [EMBASE: 70383484] - PubMed
-
- Palmer SM, Limaye AP, Banks M, Gallup D, Chapman J, Lawrence EC, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Annals of Internal Medicine 2010;152(12):761-9. [MEDLINE: ] - PubMed
Pavlopoulou 2005 Kidney {published data only}
-
- Pavlopoulou ID, Syriopoulou VP, Chelioti H, Daikos GL, Stamatiades D, Kostakis A, et al. A comparative randomised study of valacyclovir vs. oral ganciclovir for cytomegalovirus prophylaxis in renal transplant recipients. Clinical Microbiology & Infection 2005;11(9):736-43. [MEDLINE: ] - PubMed
Paya 2004 All {published data only}
-
- Boivin G, Goyette N, Gilbert C, Roberts N, Macey K, Paya C, et al. Absence of cytomegalovirus-resistance mutations after valganciclovir prophylaxis, in a prospective multicenter study of solid-organ transplant recipients. Journal of Infectious Diseases 2004;189(9):1615-8. [MEDLINE: ] - PubMed
-
- Freeman RB, Macey K, Paya C, Pescovitz MD, Humar A, Dominquez E, et al. Risk factors for cytomegalovirus (CMV) disease: results from a multicenter randomized trial of valganciclovir (VGC) [abstract no: 932]. American Journal of Transplantation 2003;3(Suppl 5):391. [CENTRAL: CN-00465780]
-
- Humar A, Mazzulli T, Moussa G, Razonable RR, Paya CV, Pescovitz MD, et al. Clinical utility of cytomegalovirus (CMV) serology testing in high-risk CMV D+/R- transplant recipients. American Journal of Transplantation 2005;5(5):1065-70. [MEDLINE: ] - PubMed
-
- Humar A, Paya C, Pescovitz MD, Dominguez E, Washburn K, Blumberg E, et al. Clinical utility of cytomegalovirus (CMV) viral load testing for predicting CMV disease in high risk D+/R- solid organ transplant recipients [abstract no: 1054]. American Journal of Transplantation 2003;3(Suppl 5):421. [CENTRAL: CN-01657546] - PubMed
-
- Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. American Journal of Transplantation 2004;4(4):611-20. [MEDLINE: ] - PubMed
Pouteil‐Noble 1996 Kidney {published data only}
-
- Pouteil-Noble C, Megas F, Chapuis F, Bosshard S, Colin C, Hadj-Aissa A, et al. Cytomegalovirus prophylaxis by ganciclovir followed by high-dose acyclovir in renal transplantation: A randomized, controlled trial. Transplantation Proceedings 1996;28(5):2811. [MEDLINE: ] - PubMed
-
- Pouteil-Noble C, Megas F, Chapuis F, Colul C, Bosshard S, Hadj-Aissa A, et al. Is CMV prophylaxis by ganciclovir-high dose acyclovir worthwhile in renal transplantation? A randomized, controlled, clinical and economical trial [abstract]. In: ISN XIII International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:343. [CENTRAL: CN-00509426]
Prabakaran 2020 Kidney {published data only}
-
- Prabakaran MR, Gupta KL, Sharma A, Kohli HS, Ramachandran R. Low-dose valganciclovir is as effective as the standard dose prophylaxis for cytomegalovirus in renal transplant recipients [abstract no: SUN-293]. Kidney International Reports 2020;5(3 Suppl):S320-1. [EMBASE: 2005255506]
Reischig 2005 Kidney {published and unpublished data}
-
- Kielberger L, Bouda M, Jindra P, Reischig T. Pharmacoeconomic impact of different regimens to prevent cytomegalovirus infection in renal transplant recipients. Kidney & Blood Pressure Research 2012;35(6):407-16. [MEDLINE: ] - PubMed
-
- Reischig T, Bouda M, Opatrny KJ, Treska V, Jindra P, Svecova M. Oral ganciclovir versus valacyclovir for prophylaxis of cytomegalovirus disease in renal transplant recipients [abstract no: 0355]. In: XIXth International Congress of the Transplantation Society; 2002 Aug 25-30; Miami (FL). 2002. [CENTRAL: CN-00416525] - PubMed
-
- Reischig T, Jindra P, Mares J, Cechura M, Svecova M, Opatrny KJ, et al. Valacyclovir for cytomegalovirus prophylaxis reduces the risk of acute renal allograft rejection: a randomized comparison with oral ganciclovir and deferred therapy [abstract no: P805]. Transplantation 2004;78(2 Suppl):483-4. - PubMed
-
- Reischig T, Jindra P, Mares J, Opatrny K, Cechura M, Svecova M, et al. Valacyclovir for cytomegalovirus prophylaxis reduces the risk of acute renal allograft rejection. Transplantation 2005;79(3):317-24. [MEDLINE: ] - PubMed
-
- Reischig T, Jindra P, Mares J, Opatrny K Jr, Treska V, Cechura M, et al. Valacyclovir for cytomegalovirus prophylaxis reduces the risk of acute renal allograft rejection: a randomized comparison of oral ganciclovir and valacyclovir [abstract no: 1225]. American Journal of Transplantation 2004;4(Suppl 8):493. - PubMed
Rondeau 1993 Kidney {published data only}
-
- Rondeau E, Bourgeon B, Peraldi MN, Lang P, Buisson C, Schulte KM, et al. Effect of prophylactic ganciclovir on cytomegalovirus infection in renal transplant recipients. Nephrology Dialysis Transplantation 1993;8(9):858-62. [MEDLINE: ] - PubMed
-
- Rondeau E, Bourgeon B, Peraldi MN, Lang P, Buisson C, Schulte KM, et al. Prophylaxis of CMV disease by ganciclovir (DHPG) in seronegative recipients of renal allograft from seropositive donors. Transplant International 1992;5 Suppl 1:S30-1. [PMID: ] - PubMed
-
- Rondeau E, Bourgeon B, Peraldi MN, Lang P, Buisson C, Schulte KM, et al. Prophylaxis of cytomegalovirus infections with ganciclovir in kidney transplant recipients [Prophylaxie des infections a cytomegalovirus par le ganciclovir chez les receveurs de greffe de rein]. Presse Medicale 1992;21(41):1979-80. [MEDLINE: ] - PubMed
Rostaing 1994 Kidney {published data only}
-
- Rostaing L, Crespin A, Icart J, Lloveras JJ, Durand D, Martinet O, et al. Cytomegalovirus (CMV) prophylaxis by acyclovir in pre-transplant CMV-positive renal transplant recipients. Transplant International 1994;7 Suppl 1:S331-5. [MEDLINE: ] - PubMed
Rubin 2002 All {published data only}
-
- Rubin RH, Kemmerly SA, Conti D, Doran M, Murray BM, Neylan JF, et al. Prevention of primary cytomegalovirus disease in organ transplant recipients with oral ganciclovir or oral acyclovir prophylaxis. Transplant Infectious Disease 2000;2(3):112-7. [MEDLINE: ] - PubMed
Saliba 1993 Liver {published data only}
-
- Saliba F, Eyraud D, Samuel D, David MF, Arulnaden JL, Dussaix E, et al. Randomized controlled trial of acyclovir for the prevention of cytomegalovirus infection and disease in liver transplant recipients. Transplantation Proceedings 1993;25(1 Pt 2):1444-5. [MEDLINE: ] - PubMed
Winston 1995 Liver {published data only}
-
- Jurim O, Martin P, Winston DJ, Shackleton C, Holt C, Feller J, et al. Failure of ganciclovir prophylaxis to prevent allograft reinfection following orthotopic liver transplantation for chronic hepatitis B infection. Liver Transplantation & Surgery 1996;2(5):370-4. [MEDLINE: ] - PubMed
-
- Winston DJ, Wirin D, Shaked A, Busuttil RW. Randomised comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet 1995;346(8967):69-74. [MEDLINE: ] - PubMed
Winston 2003 Liver {published data only}
-
- Winston DJ, Busuttil RW. Randomized controlled trial of oral ganciclovir versus oral acyclovir after induction with intravenous ganciclovir for long-term prophylaxis of cytomegalovirus disease in cytomegalovirus-seropositive liver transplant recipients. Transplantation 2003;75(2):229-33. [MEDLINE: ] - PubMed
Winston 2004 Liver {published data only}
-
- Winston DJ, Busuttil RW. Randomized controlled trial of sequential intravenous and oral ganciclovir versus prolonged intravenous ganciclovir for long-term prophylaxis of cytomegalovirus disease in high-risk cytomegalovirus-seronegative liver transplant recipients with cytomegalovirus-seropositive donors. Transplantation 2004;77(2):305-8. [MEDLINE: ] - PubMed
Winston 2012 Liver {published data only}
-
- Winston DJ, Saliba F, Blumberg E, Abouljoud M, Garcia-Diaz JB, Goss JA, et al. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial [Erratum in: Am J Transplant. 2013 Feb;13(2):529]. American Journal of Transplantation 2012;12(11):3021-30. [MEDLINE: ] - PubMed
References to studies excluded from this review
Basic‐Jukic 2019 {published data only}
-
- Basic-Jukic N, Furic-Cunko V, Hudolin T, Zimak Z, Kirincich J, Kastelan Z. A comparison of different valgancyclovir formulations in the universal 6-month prophylaxis against cmv infection in renal transplant recipients: a randomized single-centre study. Prilozi Makedonska Akademija Na Naukite I Umetnostite Oddelenie Za Medicinski Nauki 2019;40(3):47-55. [MEDLINE: ] - PubMed
-
- Basic-Jukic N, Furic-Cunko V, Hudolin T, Zimak Z, Zeljko K. Comparison of different valgancyclovir formulations in universal 6-months prophylaxis of CMV infection in renal transplant recipients: a randomized single centre study [abstract no: BOS174]. Transplant International 2019;32(Suppl 2):195. [EMBASE: 632117254] - PubMed
Brennan 1997a Kidney {published data only}
-
- Brennan DC, Burton KG, Lippmann BJ, Buller RS, Keener-Gaudreault M, Lowell JA, et al. Control of cytomegalovirus associated morbidity and costs in renal transplant patients using intensive monitoring and preemptive or deferred therapy [abstract]. In: 15th Annual Meeting. American Society of Transplant Physicians (ASTP); 1996 May 10-14; Chicago (ILL). 1996.
-
- Brennan DC, Garlock KA, Lippmann BA, Buller RS, Gaudreault-Keener M, Lowell JA, et al. Control of cytomegalovirus-associated morbidity in renal transplant patients using intensive monitoring and either preemptive or deferred therapy. Journal of the American Society of Nephrology 1997;8(1):118-25. [MEDLINE: ] - PubMed
-
- Brennan DC, Garlock KA, Lippmann BJ, Buller RS, Gaudreault-Keener M, Lowell JA, et al. Polymerase chain reaction-triggered preemptive or deferred therapy to control cytomegalovirus-associated morbidity and costs in renal transplant patients. Transplantation Proceedings 1997;29(1-2):809-11. [MEDLINE: ] - PubMed
-
- Brennan DC, Storch GA, Singer GG, Lee L, Rueda J, Schnitzler MA. The prevalence of human herpesvirus-7 in renal transplant recipients is unaffected by oral or intravenous ganciclovir. Journal of Infectious Diseases 2000;181(5):1557-61. [MEDLINE: ] - PubMed
-
- Schnitzler MA, Metheney TG, Rueda JF, Woodward RS, Lowell JA, Singer GG, et al. A 3-year follow-up of pre-emptive vs deferred treatment of cytomegalovirus disease in renal transplantation. Clinical Drug Investigation 2000;19(5):367-74. [EMBASE: 30330291]
CYTOCOR 2019 {published data only}
-
- Paez-Vega A, Cantisan S, Vaquero JM, Vidal E, Luque-Pineda A, Lobo-Acosta MA, et al. Efficacy and safety of the combination of reduced duration prophylaxis followed by immuno-guided prophylaxis to prevent cytomegalovirus disease in lung transplant recipients (CYTOCOR STUDY): an open-label, randomised, non-inferiority clinical trial. BMJ Open 2019;9(8):e030648. [MEDLINE: ] - PMC - PubMed
First 1993a {published data only}
-
- First MR, Blum MR, Brennan P, Connors J, Drummer S, Lee I, et al. Pharmacokinetics of 256U87, an acyclovir prodrug in renal transplant patients [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:168. [CENTRAL: CN-01657470]
Gerna 2008 Liver {published data only}
-
- Gerna G, Lilleri D, Callegaro A, Goglio A, Cortese S, Stroppa P, et al. Prophylaxis followed by preemptive therapy versus preemptive therapy for prevention of human cytomegalovirus disease in pediatric patients undergoing liver transplantation. Transplantation 2008;86(1):163-6. [MEDLINE: ] - PubMed
Griffiths 2016 {published data only}
Ishida 2017 {published data only}
-
- Deng R, Wang Y, Maia M, Burgess T, McBride JM, Liao XC, et al. Pharmacokinetics and exposure-response analysis of RG7667, a combination of two anticytomegalovirus monoclonal antibodies, in a phase 2a randomized trial to prevent cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrobial Agents & Chemotherapy 2018;62(2):e01108-17. [MEDLINE: ] - PMC - PubMed
-
- Ishida JH, Patel A, Mehta AK, Gatault P, McBride JM, Burgess T, et al. Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrobial Agents & Chemotherapy 2017;61(2):e01794. [MEDLINE: ] - PMC - PubMed
Jarque 2020 {published data only}
-
- Jarque M, Crespo E, Melilli E, Gutierrez A, Moreso F, Guirado L, et al. Cellular immunity to predict the risk of cytomegalovirus infection in kidney transplantation: a prospective, interventional, multicenter clinical trial. Clinical Infectious Diseases 2020;71(9):2375-85. [MEDLINE: ] - PubMed
-
- Jarque M, Melilli E, Gutierez A, Moreso F, Guirado L, Manonelles A, et al. Prospective randomized trial for predicting CMV infection according to baseline CMV-specific T-cell immunity in kidney transplant patients [abstract no: 27]. American Journal of Transplantation 2017;17(Suppl 3):216. [EMBASE: 615705134]
Jung 2001 Kidney {published data only}
-
- Jung C, Engelmann E, Borner K, Offermann G. Preemptive oral ganciclovir therapy versus prophylaxis to prevent symptomatic cytomegalovirus infection after kidney transplantation. Transplantation Proceedings 2001;33(8):3621-3. [MEDLINE: ] - PubMed
-
- Offermann G, Jung C. Preemptive oral ganciclovir therapy versus prophylaxis to prevent symptomatic cytomegalovirus infection after kidney transplantation [abstract no: 1094]. In: A Transplant Odyssey; 2001 Aug 20-23; Istanbul, Turkey. 2001. - PubMed
Khoury 2006 Kidney {published data only}
-
- Brennan DC, Hardinger KL, Bohl DL, Lockwood M, Torrence S, Schuessler R, et al. Preemptive vs prophylactic valganciclovir for CMV in renal transplantation: early results from a randomized, prospective trial [abstract no: SA-FC009]. Journal of the American Society of Nephrology 2004;15(Oct):23A. [CENTRAL: CN-00583150]
-
- Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. American Journal of Transplantation 2006;6(9):2134-43. [MEDLINE: ] - PubMed
Kiser 2012 {published data only}
-
- Kiser TH, Fish DN, Zamora MR. Evaluation of valganciclovir pharmacokinetics in lung transplant recipients. Journal of Heart & Lung Transplantation 2012;31(2):159-66. [MEDLINE: ] - PubMed
Kliem 2008 Kidney {published data only}
-
- Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. American Journal of Transplantation 2008;8(5):975-83. [MEDLINE: ] - PubMed
-
- Radermacher J, Fricke L, Burg M, Mischak H, Rohde F, Kliem V. Influence of prophylactic compared with early ganciclovir treatment on graft survival in renal allograft recipients [abstract no: TH-PO025]. Journal of the American Society of Nephrology 2006;17(Abstracts):111A. [CENTRAL: CN-00653761]
Koetz 2001 kidney/Liver {published data only}
-
- Koetz AC, Delbruch R, Furtwangler A, Hufert FT, Neumann-Haefelin D, Kirste G, et al. Cytomegalovirus pp65 antigen-guided preemptive therapy with ganciclovir in solid organ transplant recipients: a prospective double-blind, placebo-controlled study. Transplantation 2001;72(7):1325-7. [MEDLINE: ] - PubMed
Marker 1980 {published data only}
-
- Marker SC, Howard RJ, Groth KE, Mastri AR, Simmons RL, Balfour HH Jr. A trial of vidarabine for cytomegalovirus infection in renal transplant patients. Archives of Internal Medicine 1980;140(11):1441-4. [MEDLINE: ] - PubMed
Mattes 2004 {published data only}
-
- Mattes FM, Hainsworth EG, Geretti AM, Nebbia G, Prentice G, Potter M, et al. A randomized, controlled trial comparing ganciclovir to ganciclovir plus foscarnet (each at half dose) for preemptive therapy of cytomegalovirus infection in transplant recipients. Journal of Infectious Diseases 2004;189(8):1355-61. [MEDLINE: ] - PubMed
Murray 1997 {published data only}
-
- Murray B, Venuto R, Stephan R, Blas S, Myers J, Amsterdam D. A randomized trial of preemptive therapy versus conventional prophylaxis and treatment of cytomegalovirus (CMV) infection [abstract]. In: 15th Annual Meeting. American Society of Transplant Physicians (ASTP); 1996 May 10-14; Chicago (ILL). 1996. [CENTRAL: CN-01657987]
-
- Murray BM, Blas S. Cost comparison of two approaches to the management of CMV infection in renal transplant recipients [abstract no: A3235]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):695A. [CENTRAL: CN-00446876]
NCT00566072 {published data only}
-
- Devolder I. The influence of intensive education and coaching on compliance for oral ganciclovir in the prophylaxis of CMV: an open randomised trial. www.clinicaltrials.gov/ct2/show/NCT00566072 (first received 3 December 2007).
Padulles 2016 {published data only}
-
- Padulles A, Colom H, Bestard O, Cruzado JM, Melilli E, Sabe N, et al. Contribution of population pharmacokinetics to dose optimization of ganciclovir/ valganciclovir in solid organ transplant patients [abstract no: O267]. Transplant International 2015;28(Suppl 4):99. [EMBASE: 72111509] - PMC - PubMed
-
- Padulles A, Colom H, Bestard O, Torras J, Cerezo G, Rigo R, et al. Contribution of population pharmacokinetics to guide ganciclovir/valganciclovir dosage in kidney transplant patients [abstract no: FP812]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii348. [EMBASE: 72207229]
-
- Padulles A, Colom H, Caldes A, Cerezo G, Bestard O, Melilli E, et al. Ganciclovir/valganciclovir dose monitoring in solid organ transplanted patients using Bayesian prediction [abstract no: D2362]. Transplantation 2014;98(Suppl 1):762-3. [EMBASE: 71546141]
Papanicolaou 2018 {published data only}
-
- Papanicolaou GA, Silveira FP, Langston A, Pereira MR, Avery R, Wijatyk A, et al. Maribavir for treatment of cytomegalovirus (CMV) infections resistant or refractory to ganciclovir or foscarnet in hematopoietic stem cell transplant (SCT) or solid organ transplant (SOT) recipients: a randomized, dose-ranging, double-blind, phase 2 study [abstract no: 78]. Open forum Infectious Diseases 2016;1(Suppl 1). [EMBASE: 627784044]
-
- Papanicolaou GA, Silveira FP, Langston AA, Pereira MR, Avery RK, Uknis M, et al. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: a randomized, dose-ranging, double-blind, phase 2 study. Clinical Infectious Diseases 2019;68(8):1255-64. [MEDLINE: ] - PMC - PubMed
-
- Papanicolaou GA, Silveira FP, Langston AA, Pereira MR, Avery RK, Wijatyk A, et al. Maribavir for treatment of cytomegalovirus infections resistant or refractory to ganciclovir or foscarnet in hematopoietic stem cell transplant or solid organ transplant recipients: a randomized, dose-ranging, double-blind, phase 2 study [abstract no: 45]. Biology of Blood & Marrow Transplantation 2017;23(3 Suppl):S51-2. [EMBASE: 619101360]
-
- Pereira M, Silveira F, Papanicolaou G, Langston A, Avery R, Wijatyk A, et al. Maribavir for treatment of cytomegalovirus infections resistant or refractory to ganciclovir or foscarnet in solid organ transplant recipients: a phase 2 study [abstract no: 562]. American Journal of Transplantation 2017;17(Suppl 3):405. [EMBASE: 615705851]
Paya 2002 Liver {published data only}
-
- Paya CV, Wilson JA, Espy MJ, Sia IG, DeBernardi MJ, Smith TF, et al. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. Journal of Infectious Diseases 2002;185(7):854-60. [MEDLINE: ] - PubMed
-
- Razonable RR, Cruijsen H, Brown RA, Wilson JA, Harmsen WS, Wiesner RH, et al. Dynamics of cytomegalovirus replication during preemptive therapy with oral ganciclovir. Journal of Infectious Diseases 2003;187(11):1801-8. [MEDLINE: ] - PubMed
Pescovitz 2009 {published data only}
-
- Pescovitz MD, Bloom R, Pirsch J, Johnson J, Gelone S, Villano S. Pharmacokinetics (PK) and safety of maribavir (MBV) in combination with tacrolimus (TAC) in stable renal transplant recipients [abstract no: 200]. American Journal of Transplantation 2009;9(Suppl 2):248. [EMBASE: 70010073] - PubMed
-
- Pescovitz MD, Bloom R, Pirsch J, Johnson J, Gelone S, Villano SA. A randomized, double-blind, pharmacokinetic study of oral maribavir with tacrolimus in stable renal transplant recipients. American Journal of Transplantation 2009;9(10):2324-30. [MEDLINE: ] - PubMed
PROTECT 2010 {published data only}
-
- Grigioni F, Perciaccante B, Chiereghin A, Lazzarotto T, Potena L, Masetti M, et al. Efficacy and tolerability of strategies to reduce cytomegalovirus infection onset: Insights from the randomized study PROTECT [abstract no: BO408]. Transplant International 2015;28(Suppl 4):266-7. [EMBASE: 72111999]
Queiroga 2003 Kidney {published data only}
-
- Quieroga M, Castro MC, Araujo LM, Alves CF, Kakehashi E, Panutti C, et al. A prospective, randomized controlled trial comparing oral ganciclovir with weekly monitored CMV-antigenemia in renal transplant patients with a high-risk for CMV infection [abstract no: 1403]. American Journal of Transplantation 2003;3(Suppl 5):511. [CENTRAL: CN-00447308]
Rayes 2001 Liver {published data only}
-
- Rayes N, Seehofer D, Oettle H, Schmidt CA, Neuhaus R, Steinmuller T, et al. Prospective randomised trial to assess the value of preemptive oral therapy for CMV-infection after OLT [abstract no: PO989]. In: XVIII International Congress of the Transplantation Society; 2000 Aug 27-Sept 1; Rome, Italy. 2000. [CENTRAL: CN-00416522]
-
- Rayes N, Seehofer D, Schmidt CA, Oettle H, Muller AR, Steinmuller T, et al. Prospective randomized trial to assess the value of preemptive oral therapy for CMV infection following liver transplantation. Transplantation 2001;72(5):881-5. [MEDLINE: ] - PubMed
-
- Struecker B, Seehofer D, Andreas A, Neuhaus P, Nada R. Impact of CMV viremia after liver transplantation: 15 year results of a prospective, randomized clinical trial [abstract no: D2399]. Transplantation 2014;98(Suppl 1):773. [EMBASE: 71546178]
Reischig 2008 Kidney {published data only}
-
- Kielberger L, Bouda M, Jindra P, Reischig T. Pharmacoeconomic impact of different regimens to prevent cytomegalovirus infection in renal transplant recipients. Kidney & Blood Pressure Research 2012;35(6):407-16. [MEDLINE: ] - PubMed
-
- Reischig T, Hribova P, Jindra P, Hes O, Bouda M, Treska V, et al. Improved long-term renal allograft survival in preemptive valganciclovir therapy compared to valacyclovir prophylaxis for cytomegalovirus: Results of randomized controlled trial [abstract no: 1464]. American Journal of Transplantation 2011;11(Suppl 2):455. [EMBASE: 70406517]
-
- Reischig T, Hribova P, Jindra P, Hes O, Bouda M, Treska V, et al. Improved long-term renal allograft survival in preemptive valganciclovir therapy compared to valacyclovir prophylaxis for cytomegalovirus: results of randomized controlled trial [abstract no: O-145]. Transplant International 2011;24(Suppl 2):40. [EMBASE: 70527222]
-
- Reischig T, Hribova P, Jindra P, Hes O, Bouda M, Treska V, et al. Long-term outcomes of pre-emptive valganciclovir compared with valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation. Journal of the American Society of Nephrology 2012;23(9):1588-97. [MEDLINE: ] - PMC - PubMed
-
- Reischig T, Jindra P, Hes O, Svecova M, Klaboch J, Treska V. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. American Journal of Transplantation 2008;8(1):69-77. [MEDLINE: ] - PubMed
Sagedal 2003 Kidney {published data only}
-
- Sagedal S, Nordal KP, Hartmann A, Midvedt K, Foss A, Asberg A, et al. Pre-emptive therapy of CMVpp65 antigen positive renal transplant recipients with oral ganciclovir: a randomized, comparative study. Nephrology Dialysis Transplantation 2003;18(9):1899-908. [MEDLINE: ] - PubMed
Scott 2011 Liver {published data only}
-
- Scott GM, Naing Z, Pavlovic J, Iwasenko JM, Angus P, Jones R, et al. Viral factors influencing the outcome of human cytomegalovirus infection in liver transplant recipients. Journal of Clinical Virology 2011;51(4):229-33. [MEDLINE: ] - PubMed
-
- Scott GM, Naing Z, Pavlovic J, Waliuzzaman ZM, Iwasenko J, Rismanto N, et al. CMV infection and antiviral resistance in a liver transplant cohort followed longitudinally [abstract o: 1945]. Transplantation 2010;90(Suppl 1):668. [EMBASE: 71532382]
Singh 1994 Liver {published data only}
-
- Sing N, Yu VL, Mieles L, Wagener MM, Miner RC, Gayowsky T. High-dose acyclovir compared with short-course preemptive ganciclovir therapy to prevent cytomegalovirus disease in liver transplant recipients. Annals of Internal Medicine 1994;120(5):375-81. [MEDLINE: ] - PubMed
Singh 2000 Liver {published data only}
-
- Singh N, Paterson DL, Gayowsky T, Wagener MM, Marino IR. Cytomegalovirus antigenemia directed pre-emptive prophylaxis with oral versus iv ganciclovir for the prevention of cytomegalovirus disease in liver transplant recipients. Transplantation 2000;70(5):717-22. [MEDLINE: ] - PubMed
-
- Singh N, Yu VL, Gayowski T, Marino IR. CMV antigenemia directed preemptive prophylaxis with oral ganciclovir for the prevention of CMV disease in liver transplant recipients: a prospective, randomised, controlled trial [abstract no: 442]. Transplantation 1998;65(12):S113. [CENTRAL: CN-00764233] - PubMed
Singh 2019 Liver {published data only}
-
- Singh N, Winston D, Razonable RR, Lyon GM, Silveira FP, Wagener M, et al. Preemptive therapy (PET) vs. prophylaxis for prevention of cytomegalovirus (CMV) disease in high-risk donor seropositive/recipient seronegative (D+R-) liver transplant recipients (LTR): A NIH-sponsored, randomized, controlled, multicenter trial [abstract no: LB21]. Open Forum Infectious Diseases 2018;5(Suppl 1):S766. [EMBASE: 629442710]
-
- Singh N, Winston DJ, Razonable RR, Lyon GM, Silveira FP, Wagener MM, et al. Cost-effectiveness of preemptive therapy versus prophylaxis in a randomized clinical trial for the prevention of CMV disease in seronegative liver transplant recipients with seropositive donors. Clinical Infectious Diseases 2020;73(9):e2739-45. [MEDLINE: ] - PMC - PubMed
-
- Singh N, Winston DJ, Razonable RR, Lyon GM, Silveira FP, Wagener MM, et al. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors: a randomized clinical trial. JAMA 2020;323(14):1378-87. [MEDLINE: ] - PMC - PubMed
-
- Singh N, Winston DJ, Razonable RR, Lyon GM, Silveira FP, Wagener MM, et al. Risk factors for cytomegalovirus viremia following liver transplantation with a seropositive donor and seronegative recipient receiving antiviral therapy. Journal of Infectious Diseases 2021;223(6):1073-7. [MEDLINE: ] - PMC - PubMed
-
- Wagener MM, Winston DJ, Razonable RR, Lyon GM, Silveira FP, Limaye AP, et al. Preemptive therapy is cost effective when compared to prophylaxis in cytomegalovirus donor positive-recipient negative liver transplant recipients [abstract no: 111). American Journal of Transplantation 2019;1(3):461. [EMBASE: 628454976]
VICTOR 2007 {published data only}
-
- Asberg A, Humar A, Jardine AG, Rollag H, Pescovitz MD, Mouas H, et al. Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. American Journal of Transplantation 2009;9(5):1205-13. [MEDLINE: ] - PubMed
-
- Asberg A, Humar A, Rollag H, Jardine AG, Kumar D, Aukrust P, et al. Lessons learned from a randomized study of oral valganciclovir versus parenteral ganciclovir treatment of cytomegalovirus disease in solid organ transplant recipients: The VICTOR Trial. Clinical Infectious Diseases 2016;62(9):1154-60. [MEDLINE: ] - PubMed
-
- Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. American Journal of Transplantation 2007;7(9):2106-13. [MEDLINE: ] - PubMed
-
- Asberg A, Jardine AG, Bignamini AA, Rollag H, Pescovitz MD, Gahlemann CC, et al. Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients. American Journal of Transplantation 2010;10(8):1881-8. [MEDLINE: ] - PubMed
-
- Asberg A, Pescovitz MD, Humar A, Jardine AG, Rollag H, Mouas H, et al. Oral valganciclovir and intravenous ganciclovir results in comparable long-term outcomes in transplant recipients with CMV disease: the VICTOR study [abstract no: 634]. Transplantation 2008;86(2S):222. [CENTRAL: CN-00740534]
Westall 2018 {published data only}
-
- Westall GP, Cristiano Y, Levvey BJ, Whitford H, Paraskeva MA, Paul E, et al. A randomized study of quantiferon-CMV-directed versus fixed-duration valganciclovir prophylaxis to reduce late CMV following lung transplantation. Transplantation 2019;103(5):1005-13. [MEDLINE: ] - PubMed
Witzke 2012 Kidney {published data only}
-
- Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 2012;93(1):61-8. [MEDLINE: ] - PubMed
-
- Witzke O, Nitschke M, Bartels M, Ott U, Hauser IA. CMV valganciclovir prophylaxis versus preemptive therapy after renal transplantation: one year results of a randomized clinical trial [abstract no: 3273]. Transplantation 2010;90(Suppl 2):186. [EMBASE: 71531461]
-
- Witzke O, Nitschke M, Bartels M, Wolters H, Wolf G, Reinke P, et al. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: long-term results after 7 years of a randomized clinical trial. Transplantation 2018;102(5):876-82. [MEDLINE: ] - PubMed
-
- Witzke O. CMV valganciclovir prophylaxis versus preemptive therapy after renal transplantation: Two year results of a randomized clinical trial [abstract no: LB05]. American Journal of Transplantation 2011;11(Suppl 2):210. [EMBASE: 70405637]
-
- Witzke O. CMV valganciclovir prophylaxis versus preemptive therapy after renal transplantation: two year results of a randomized clinical trial [abstract no: P042]. Transplant International 2011;24(Suppl 3):31-2. [EMBASE: 70537366]
Yang 1998 Kidney {published data only}
-
- Yang CW, An HJ, Kim YO, Shin YS, Chang YS, Bang BK. Indication of ganciclovir treatment during early cytomegalovirus (CMV) viremia in CMV seropositive recipients. a longitudinal study of CMV pp65 antigenemia (Ag) assay [abstract no: A3404]. Journal of the American Society of Nephrology 1996;7(9):1928. [CENTRAL: CN-00775192]
-
- Yang CW, Kim YO, Kin YS, Kin SY, Moon IS, Ahn HJ, et al. Clinical course of cytomegalovirus (CMV) viremia with and without ganciclovir treatment in CMV-seropositive kidney transplant recipients. Longitudinal follow-up of CMV pp65 antigenemia assay. American Journal of Nephrology 1998;18(5):373-8. [MEDLINE: ] - PubMed
References to studies awaiting assessment
Limaye 2023 {published data only}
-
- Limaye AP, Budde K, Humar A, Garcia-Diaz J, Carroll RP, Murata Y, et al. Safety and efficacy of letermovir (LET) versus valganciclovir (VGCV) for prevention of cytomegalovirus (CMV) disease in kidney transplant recipients (KTRs): a phase 3 randomized study [abstract no: LB2307]. Open Forum Infectious Diseases 2022;9(Suppl 2):S936. [EMBASE: 640022298]
Verghese 2022 {published data only}
-
- Verghese PS, Matas A, Chinnakotla S, Evans M, Balfour H Jr. Post-kidney transplant valacyclovir versus valganciclovir for CMV prophylaxis: a randomized controlled trial [abstract no: 51]. American Journal of Transplantation 2022;22(Suppl 3):354. [EMBASE: 639185621]
References to ongoing studies
NCT04225923 {published data only}
-
- NCT04225923. A study for kidney transplant recipients at high-risk of cytomegalovirus infection [A phase 2, randomized, double-blind, placebo-controlled study of NPC-21 for kidney transplant recipients at high-risk of cytomegalovirus infection]. www.clinicaltrials.gov/study/NCT04225923 (first posted 13 January 2020).
Additional references
Baliga 2004
-
- Baliga RS, Kadambi PV, Javaid B, Harland R, Williams JW, Thistlethwaite JR, et al. A nationwide survey of cytomegalovirus prophylaxis and treatment in the transplant community [abstract]. American Journal of Transplantation 2004;4(Suppl 8):495.
Basgoz 1995
-
- Basgoz N, Preiksaitis J. Post-transplant lymphoproliferative disorder. Infectious Diseases Clinics of North America 1995;9(4):901-23. [MEDLINE: ] - PubMed
Bate 2010
Camacho‐Gonzalez 2011
Couchoud 1998a
Couchoud 1998b
-
- Couchoud C, Cucherat M, Haugh M, Pouteil-Noble C. Cytomegalovirus prophylaxis with antiviral agents in solid organ transplantation: a meta-analysis. Transplantation 1998;65(5):641-7. [MEDLINE: ] - PubMed
Emery 2000
-
- Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000;355(9220):2032-6. [MEDLINE: ] - PubMed
Fiddian 2002
-
- Fiddian P, Sabin CA, Griffiths PD. Valacyclovir provides optimum acyclovir exposure for prevention of cytomegalovirus and related outcomes after organ transplantation. Journal of Infectious Diseases 2002;186 Suppl 1:S110-5. [MEDLINE: ] - PubMed
Fishman 1995
-
- Fishman JA. Pneumocystis carinii and parasitic infections in transplantation. Infectious Disease Clinics of North America 1995;9(4):1005-44. [MEDLINE: ] - PubMed
Fishman 1998
-
- Fishman JA, Rubin RH. Infection in organ-transplant recipients. New England Journal of Medicine 1998;338(24):1741-51. [MEDLINE: ] - PubMed
Florescu 2014
-
- Florescu DF, Qiu F, Schmidt CM, Kalil AC. A direct and indirect comparison meta-analysis on the efficacy of cytomegalovirus preventive strategies in solid organ transplant. Clinical Infectious Diseases 2014;58(6):785-803. [PMID: ] - PubMed
George 1997
-
- George MJ, Snydman DR, Werner BG, Griffith J, Falagas ME, Dougherty NN, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. American Journal of Medicine 1997;103(2):106-13. [MEDLINE: ] - PubMed
Gourishankar 2001
-
- Gourishankar S, Wong W, Dorval M. Meta-analysis of prophylaxis of CMV disease in solid organ transplantation: is Ganciclovir a superior agent to acyclovir? Transplantation Proceedings 2001;33(1-2):1870-2. [MEDLINE: ] - PubMed
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] - PubMed
Grattan 1989
-
- Grattan MT, Moreno-Cabral CE, Starnes BA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA 1989;261(24):3561-6. [MEDLINE: ] - PubMed
Hadley 1995
-
- Hadley S, Karchmer AW. Fungal infections in solid organ transplant recipients. Infectious Disease Clinics of North America 1995;9(4):1045-74. [MEDLINE: ] - PubMed
Hakimi 2017
-
- Hakimi Z, Aballéa S, Ferchichi S, Scharn M, Odeyemi IA, Toumi M, et al. Burden of cytomegalovirus disease in solid organ transplant recipients: a national matched cohort study in an inpatient setting. Transplant Infectious Disease 2017;19(5):e12732. [PMID: ] - PubMed
Hartmann 2006
-
- Hartmann A, Sagedal S, Hjelmesaeth J. The natural course of cytomegalovirus infection and disease in renal transplant recipients. Transplantation 2006;82(2 Suppl):S15-7. [PMID: ] - PubMed
Hellemans 2022
-
- Hellemans R, Abramowicz D. Cytomegalovirus after kidney transplantation in 2020: moving towards personalized prevention. Nephrology Dialysis Transplantation 2022;37(5):810-16. [PMID: ] - PubMed
Higgins 2003
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et a l. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hodson 2007
-
- Hodson EM, Jones CA, Strippoli GF, Webster AC, Craig JC. Immunoglobulins, vaccines or interferon for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD005129. [DOI: 10.1002/14651858.CD005129.pub2] - DOI - PubMed
Humar 2009
-
- Humar A, Snydman D. Cytomegalovirus in solid organ transplant recipients. American Journal of Transplantation 2009;9 Suppl 4:S78-86. [EMBASE: 20070700] - PubMed
Jassal 1998
-
- Jassal SV, Roscoe JM, Zaltzman JS, Mazzulli T, Krajden M, Gadawski M, et al. Clinical practice guidelines: prevention of cytomegalovirus disease after renal transplantation. Journal of the American Society of Nephrology 1998;9(9):1697-708. [MEDLINE: ] - PubMed
Kalil 2005
-
- Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Annals of Internal Medicine 2005;143(12):870-80. [MEDLINE: ] - PubMed
KDIGO 2009
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Chapter 13.2: Cytomegalovirus. American Journal of Transplantation 2009;9(Suppl 3):S46-8. [MEDLINE: ] - PubMed
KDIGO Transplant Working Group 2009
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1-155. [PMID: ] - PubMed
Keenan 1991
-
- Keenan RJ, Lega ME, Dummer JS, Paradis IL, Dauber JH, Rabinowich H, et al. Cytomegalovirus serologic status and postoperative infection correlated with risk of developing chronic rejection after pulmonary transplantation. Transplantation 1991;51(2):433-8. [MEDLINE: ] - PubMed
Kotton 2013
-
- Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013;96(4):333-60. [PMID: ] - PubMed
Kotton 2018
-
- Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018;102(6):900-31. [PMID: ] - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine / Public Library of Science 2009;6(7):e1000100. [PMID: ] - PMC - PubMed
Linden 2000
-
- Linden PK. Infections in liver transplant recipients. In: Glauser MP, Pizzo PA, editors(s). Management of Infections in Immunocompromised Patients. 1st edition. United Kingdom: WB Saunders, 2000:295-322.
Michaels 2000
-
- Michaels M, Green M. Infections in lung and heart-lung transplant recipients. In: Glauser MP, Pizzo PA, editors(s). Management of Infections in Immunocompromised Patients. 1st edition. United Kingdom: WB Saunders, 2000:363-93.
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [MEDLINE: ] - PubMed
Owers 2013
-
- Owers DS, Webster AC, Strippoli GF, Kable K, Hodson EM. Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD005133. [DOI: 10.1002/14651858.CD005133.pub3] - DOI - PMC - PubMed
Pascual 2018
Raval 2020
-
- Raval AD, Kistler K, Tang Y, Murata Y, Snydman DR. Antiviral treatment approaches for cytomegalovirus prevention in kidney transplant recipients: a systematic review of randomized controlled trials. Transplantation Reviews 2020;35(1):100587. [PMID: ] - PubMed
Razonable 2013
-
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. American Journal of Transplantation 2013;13 Suppl 4:93-106. [PMID: ] - PubMed
Razonable 2019
-
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clinical Transplantation 2019;33(9):e13512. [PMID: ] - PubMed
Rubin 1989
-
- Rubin RH. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA 1989;261(24):3607-9. [MEDLINE: ] - PubMed
Rubin 2000
-
- Rubin RH, Rosenberg E. Infection in solid organ transplant: an introduction. In: Glauser MP, Pizzo PA, editors(s). Management of Infections in Immunocompromised Patients. 1st edition. United Kingdom: WB Saunders, 2000:246-65.
Sagedal 2004
-
- Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney International 2004;66(1):329-37. [PMID: ] - PubMed
Santesso 2020
-
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [PMID: ] - PubMed
Schultz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [MEDLINE: ] - PubMed
Schünemann 2022a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Schünemann 2022b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sommerer 2019
-
- Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Witzke O, et al. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney International 2019;96(1):231-44. [PMID: ] - PubMed
Strippoli 2006a
Strippoli 2006b
-
- Strippoli GF, Hodson EM, Jones CA, Craig JC. Preemptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation 2006;81(2):139-45. [MEDLINE: ] - PubMed
Valentine 1999
-
- Valantine HA. Role of CMV in transplant coronary artery disease and survival after heart transplantation. Transplant Infectious Disease 1999;1 Suppl 1:25-30. [MEDLINE: ] - PubMed
Van den Berg 1996
-
- Van den Berg AP, Klompmaker IJ, Haagsma EB, Peeters PM, Meerman L, Verwer R, et al. Evidence for increased rate of bacterial infections in liver transplant patients with cytomegalovirus infection. Clinical Transplantation 1996;10(2):224-31. [MEDLINE: ] - PubMed
Van der Bij 2001
-
- Van der Bij W, Speich R. Management of cytomegalovirus infection and disease after solid-organ transplantation. Clinical Infectious Diseases 2001;33 Suppl 1:32-7. [MEDLINE: ] - PubMed
Zuhair 2019
-
- Zuhair M, Smit GS, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Reviews in Medical Virology 2019;29(3):e2034. [PMID: ] - PubMed
References to other published versions of this review
Hodson 2005a
Hodson 2005b
Hodson 2005c
-
- Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay BG, Kable K, et al. Antiviral medications to prevent cytomegalovirus disease and early death in solid-organ transplant recipients: a systematic review of randomised controlled trials. Lancet 2005;365(9477):2105-15. [MEDLINE: ] - PubMed
Hodson 2008
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

