Effects of the selective AMPA modulator NBI-1065845 on the pharmacokinetics of midazolam or ethinyl estradiol-levonorgestrel in healthy adults
- PMID: 38700236
- PMCID: PMC11067504
- DOI: 10.1111/cts.13791
Effects of the selective AMPA modulator NBI-1065845 on the pharmacokinetics of midazolam or ethinyl estradiol-levonorgestrel in healthy adults
Abstract
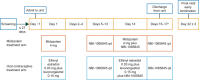
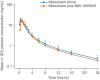
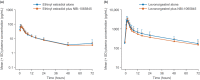
This parallel-arm, phase I study investigated the potential cytochrome P450 (CYP)3A induction effect of NBI-1065845 (TAK-653), an investigational α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor potentiator in phase II development for major depressive disorder. The midazolam treatment arm received the sensitive CYP3A substrate midazolam on Day 1, followed by NBI-1065845 alone on Days 5-13; on Day 14, NBI-1065845 was administered with midazolam, then NBI-1065845 alone on Day 15. The oral contraceptive treatment arm received ethinyl estradiol-levonorgestrel on Day 1, then NBI-1065845 alone on Days 5-13; on Day 14, NBI-1065845 was administered with ethinyl estradiol-levonorgestrel, then NBI-1065845 alone on Days 15-17. Blood samples were collected for pharmacokinetic analyses. The midazolam treatment arm comprised 14 men and 4 women, of whom 16 completed the study. Sixteen of the 17 healthy women completed the oral contraceptive treatment arm. After multiple daily doses of NBI-1065845, the geometric mean ratios (GMRs) (90% confidence interval) for maximum observed concentration were: midazolam, 0.94 (0.79-1.13); ethinyl estradiol, 1.00 (0.87-1.15); and levonorgestrel, 0.99 (0.87-1.13). For area under the plasma concentration-time curve (AUC) from time 0 to infinity, the GMRs were as follows: midazolam, 0.88 (0.78-0.98); and ethinyl estradiol, 1.01 (0.88-1.15). For levonorgestrel, the GMR for AUC from time 0 to the last quantifiable concentration was 0.87 (0.78-0.96). These findings indicate that NBI-1065845 is not a CYP3A inducer and support its administration with CYP3A substrates. NBI-1065845 was generally well tolerated, with no new safety signals observed after coadministration of midazolam, ethinyl estradiol, or levonorgestrel.
© 2024 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
S.L., A.I., J.M.‐S., M.K., M.F., and J.B.S. are employees of Neurocrine Biosciences, Inc. and own stock or share options. D.W. reports compensation for serving as a consultant or speaker, or research support, paid to the author or the institution they work for, from AbbVie, Acadia Pharmaceuticals, Alkermes, Allergan, Avanir Pharmaceuticals, Biogen, Boehringer Ingelheim, Cerevel Therapeutics, Indivior, Intra‐Cellular Therapies, Johnson & Johnson Pharmaceutical Research & Development, Janssen Pharmaceuticals, Karuna Therapeutics, Lundbeck, Lupin, Lyndra Therapeutics, Neurocrine Biosciences, Novartis, Noven Pharmaceuticals, Otsuka Pharmaceutical, Pfizer, Roche, Sunovion and Takeda.
Figures
References
-
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163:1905‐1917. - PubMed
-
- Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress. Anxiety. 2009;26:83‐97. - PubMed
-
- Suzuki A, Hara H, Kimura H. Role of the AMPA receptor in antidepressant effects of ketamine and potential of AMPA receptor potentiators as a novel antidepressant. Neuropharmacology. 2023;222:109308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

