Novel pentafluorosulfanyl-containing triclocarban analogs selectively kill Gram-positive bacteria
- PMID: 38700321
- PMCID: PMC11237694
- DOI: 10.1128/spectrum.00071-24
Novel pentafluorosulfanyl-containing triclocarban analogs selectively kill Gram-positive bacteria
Abstract
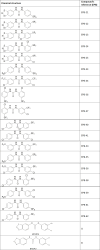
Novel antimicrobial agents are needed to combat antimicrobial resistance. This study tested novel pentafluorosulfanyl-containing triclocarban analogs for their potential antibacterial efficacy. Standard procedures were used to produce pentafluorosulfanyl-containing triclocarban analogs. Twenty new compounds were tested against seven Gram-positive and Gram-negative indicator strains as well as 10 clinical isolates for their antibacterial and antibiofilm activity. Mechanistic investigations focused on damage to cell membrane, oxidizing reduced thiols, iron-sulfur clusters, and oxidative stress to explain the compounds' activity. Safety profiles were assessed using cytotoxicity experiments in eukaryotic cell lines. Following screening, selected components had significantly better antibacterial and antibiofilm activity against Gram-positive bacteria in lower concentrations in comparison to ciprofloxacin and gentamycin. For instance, one compound had a minimum inhibitory concentration of <0.0003 mM, but ciprofloxacin had 0.08 mM. Mechanistic studies show that these novel compounds do not affect reduced thiol content, iron-sulfur clusters, or hydrogen peroxide pathways. Their impact comes from Gram-positive bacterial cell membrane damage. Tests on cell culture toxicity and host component safety showed promise. Novel diarylurea compounds show promise as Gram-positive antimicrobials. These compounds offer prospects for study and optimization.
Importance: The rise of antibiotic resistance among bacterial pathogens poses a significant threat to global health, underscoring the urgent need for novel antimicrobial agents. This study presents research on a promising class of novel compounds with potent antibacterial properties against Gram-positive bacteria, notably Staphylococcus aureus and MRSA. What sets these novel analogs apart is their superior efficacy at substantially lower concentrations compared with commonly used antibiotics like ciprofloxacin and gentamycin. Importantly, these compounds act by disrupting the bacterial cell membrane, offering a unique mechanism that could potentially circumvent existing resistance mechanisms. Preliminary safety assessments also highlight their potential for therapeutic use. This study not only opens new avenues for combating antibiotic-resistant infections but also underscores the importance of innovative chemical approaches in addressing the global antimicrobial resistance crisis.
Keywords: Gram-positives; MRSA; Staphylococcus aureus; antibacterial; antibiofilm; diarylurea scaffold; novel antimicrobials; pentafluorosulfanyl.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- Natural Sciences Engineering Research Council of Canada/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- PID2021-122116OB-I00/EC | European Regional Development Fund (ERDF)
- Canada Research Chair/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

