Preclinical validation of an Escherichia coli O-antigen glycoconjugate for the prevention of serotype O1 invasive disease
- PMID: 38700324
- PMCID: PMC11237799
- DOI: 10.1128/spectrum.04213-23
Preclinical validation of an Escherichia coli O-antigen glycoconjugate for the prevention of serotype O1 invasive disease
Abstract
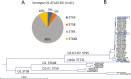
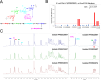
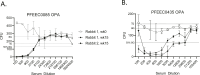
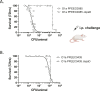
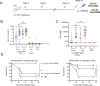
A US collection of invasive Escherichia coli serotype O1 bloodstream infection (BSI) isolates were assessed for genotypic and phenotypic diversity as the basis for designing a broadly protective O-antigen vaccine. Eighty percent of the BSI isolate serotype O1 strains were genotypically ST95 O1:K1:H7. The carbohydrate repeat unit structure of the O1a subtype was conserved in the three strains tested representing core genome multi-locus sequence types (MLST) sequence types ST95, ST38, and ST59. A long-chain O1a CRM197 lattice glycoconjugate antigen was generated using oxidized polysaccharide and reductive amination chemistry. Two ST95 strains were investigated for use in opsonophagocytic assays (OPA) with immune sera from vaccinated animals and in murine lethal challenge models. Both strains were susceptible to OPA killing with O1a glycoconjugate post-immune sera. One of these, a neonatal sepsis strain, was found to be highly lethal in the murine challenge model for which virulence was shown to be dependent on the presence of the K1 capsule. Mice immunized with the O1a glycoconjugate were protected from challenges with this strain or a second, genotypically related, and similarly virulent neonatal isolate. This long-chain O1a CRM197 lattice glycoconjugate shows promise as a component of a multi-valent vaccine to prevent invasive E. coli infections.
Importance: The Escherichia coli serotype O1 O-antigen serogroup is a common cause of invasive bloodstream infections (BSI) in populations at risk such as newborns and the elderly. Sequencing of US BSI isolates and structural analysis of O polysaccharide antigens purified from strains that are representative of genotypic sub-groups confirmed the relevance of the O1a subtype as a vaccine antigen. O polysaccharide was purified from a strain engineered to produce long-chain O1a O-antigen and was chemically conjugated to CRM197 carrier protein. The resulting glycoconjugate elicited functional antibodies and was protective in mice against lethal challenges with virulent K1-encapsulated O1a isolates.
Keywords: Escherichia coli; K1 capsule; O-antigen; glycoconjugate vaccine.
Conflict of interest statement
All authors were Pfizer employees during the study and may be shareholders of the company. R.G.K.D., A.S.A., L.C., S.K., R.P., and L.C. are inventors on a related patent.
Figures
Similar articles
-
A cynomolgus monkey E. coli urinary tract infection model confirms efficacy of new FimH vaccine candidates.Infect Immun. 2024 Oct 15;92(10):e0016924. doi: 10.1128/iai.00169-24. Epub 2024 Sep 19. Infect Immun. 2024. PMID: 39297649 Free PMC article.
-
Preclinical Immunogenicity and Efficacy of Optimized O25b O-Antigen Glycoconjugates To Prevent MDR ST131 E. coli Infections.Infect Immun. 2022 Apr 21;90(4):e0002222. doi: 10.1128/iai.00022-22. Epub 2022 Mar 21. Infect Immun. 2022. PMID: 35311580 Free PMC article.
-
A bivalent vaccine derived from attenuated Salmonella expressing O-antigen polysaccharide provides protection against avian pathogenic Escherichia coli O1 and O2 infection.Vaccine. 2018 Feb 14;36(8):1038-1046. doi: 10.1016/j.vaccine.2018.01.036. Epub 2018 Jan 19. Vaccine. 2018. PMID: 29358057
-
A review: relation between invasiveness and the K1 capsular polysaccharide of Escherichia coli.Pediatr Res. 1976 Feb;10(2):82-7. doi: 10.1203/00006450-197602000-00002. Pediatr Res. 1976. PMID: 1107953 Review.
-
Impact and Control of Sugar Size in Glycoconjugate Vaccines.Molecules. 2022 Sep 29;27(19):6432. doi: 10.3390/molecules27196432. Molecules. 2022. PMID: 36234967 Free PMC article. Review.
Cited by
-
A cynomolgus monkey E. coli urinary tract infection model confirms efficacy of new FimH vaccine candidates.Infect Immun. 2024 Oct 15;92(10):e0016924. doi: 10.1128/iai.00169-24. Epub 2024 Sep 19. Infect Immun. 2024. PMID: 39297649 Free PMC article.
References
-
- Powell EC, Mahajan PV, Roosevelt G, Hoyle JD, Gattu R, Cruz AT, Rogers AJ, Atabaki SM, Jaffe DM, Casper TC, Ramilo O, Kuppermann N, Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) . 2018. Epidemiology of bacteremia in febrile infants aged 60 days and younger. Ann Emerg Med 71:211–216. doi:10.1016/j.annemergmed.2017.07.488 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

