Assessment of the hypothetical protein BB0616 in the murine infection of Borrelia burgdorferi
- PMID: 38700336
- PMCID: PMC11237664
- DOI: 10.1128/iai.00090-24
Assessment of the hypothetical protein BB0616 in the murine infection of Borrelia burgdorferi
Abstract
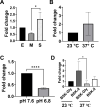
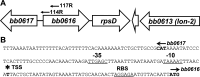
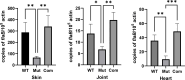
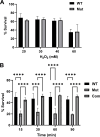
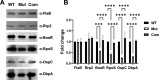
bb0616 of Borrelia burgdorferi, the Lyme disease pathogen, encodes a hypothetical protein of unknown function. In this study, we showed that BB0616 was not surface-exposed or associated with the membrane through localization analyses using proteinase K digestion and cell partitioning assays. The expression of bb0616 was influenced by a reduced pH but not by growth phases, elevated temperatures, or carbon sources during in vitro cultivation. A transcriptional start site for bb0616 was identified by using 5' rapid amplification of cDNA ends, which led to the identification of a functional promoter in the 5' regulatory region upstream of bb0616. By analyzing a bb0616-deficient mutant and its isogenic complemented counterparts, we found that the infectivity potential of the mutant was significantly attenuated. The inactivation of bb0616 displayed no effect on borrelial growth in the medium or resistance to oxidative stress, but the mutant was significantly more susceptible to osmotic stress. In addition, the production of global virulence regulators such as BosR and RpoS as well as virulence-associated outer surface lipoproteins OspC and DbpA was reduced in the mutant. These phenotypes were fully restored when gene mutation was complemented with a wild-type copy of bb0616. Based on these findings, we concluded that the hypothetical protein BB0616 is required for the optimal infectivity of B. burgdorferi, potentially by impacting B. burgdorferi virulence gene expression as well as survival of the spirochete under stressful conditions.
Keywords: Borrelia burgdorferi; Lyme disease; RpoS; pathogenesis; regulation of gene expression; virulence regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


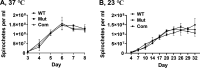
Similar articles
-
bb0689 contributes to the virulence of Borrelia burgdorferi in a murine model of Lyme disease.Infect Immun. 2025 Jan 31;93(1):e0045924. doi: 10.1128/iai.00459-24. Epub 2024 Dec 16. Infect Immun. 2025. PMID: 39679711 Free PMC article.
-
DksA plays an essential role in regulating the virulence of Borrelia burgdorferi.Mol Microbiol. 2020 Jul;114(1):172-183. doi: 10.1111/mmi.14504. Epub 2020 Apr 14. Mol Microbiol. 2020. PMID: 32227372 Free PMC article.
-
Role of the Hypothetical Protein BB0563 during Borrelia burgdorferi Infection in Animals.Infect Immun. 2023 Mar 15;91(3):e0053922. doi: 10.1128/iai.00539-22. Epub 2023 Feb 6. Infect Immun. 2023. PMID: 36744894 Free PMC article.
-
Inactivation of bb0184, which encodes carbon storage regulator A, represses the infectivity of Borrelia burgdorferi.Infect Immun. 2011 Mar;79(3):1270-9. doi: 10.1128/IAI.00871-10. Epub 2010 Dec 20. Infect Immun. 2011. PMID: 21173314 Free PMC article.
-
RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants.Infect Immun. 2004 Nov;72(11):6433-45. doi: 10.1128/IAI.72.11.6433-6445.2004. Infect Immun. 2004. PMID: 15501774 Free PMC article.
Cited by
-
bb0689 contributes to the virulence of Borrelia burgdorferi in a murine model of Lyme disease.Infect Immun. 2025 Jan 31;93(1):e0045924. doi: 10.1128/iai.00459-24. Epub 2024 Dec 16. Infect Immun. 2025. PMID: 39679711 Free PMC article.
-
Analysis of bb0556 Expression and Its Role During Borrelia burgdorferi Mammalian Infection.Mol Microbiol. 2024 Dec;122(6):831-846. doi: 10.1111/mmi.15319. Epub 2024 Sep 20. Mol Microbiol. 2024. PMID: 39305042
-
Linking genomic evolutionary transitions to ecological phenotypic adaptations in Spirochaetes.bioRxiv [Preprint]. 2025 Jul 4:2025.07.04.663154. doi: 10.1101/2025.07.04.663154. bioRxiv. 2025. PMID: 40631289 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

