Prostate-Specific Membrane Antigen Targeted StarPEG Nanocarrier for Imaging and Therapy of Prostate Cancer
- PMID: 38700450
- PMCID: PMC11281871
- DOI: 10.1002/adhm.202304618
Prostate-Specific Membrane Antigen Targeted StarPEG Nanocarrier for Imaging and Therapy of Prostate Cancer
Abstract
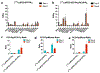
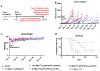
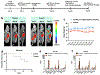
The tumor uptake of large non-targeted nanocarriers primarily occurs through passive extravasation, known as the enhanced permeability and retention (EPR) effect. Prior studies demonstrated improved tumor uptake and retention of 4-arm 40 kDa star polyethylene glycol (StarPEG) polymers for cancer imaging by adding prostate-specific membrane antigen (PSMA) targeting small molecule ligands. To test PSMA-targeted delivery and therapeutic efficacy, StarPEG nanodrugs with/without three copies of PSMA-targeting ligands, ACUPA, are designed and synthesized. For single-photon emission computed tomography (SPECT) imaging and therapy, each nanocarrier is labeled with 177Lu using DOTA radiometal chelator. The radiolabeled nanodrugs, [177Lu]PEG-(DOTA)1 and [177Lu]PEG-(DOTA)1(ACUPA)3, are evaluated in vitro and in vivo using PSMA+ PC3-Pip and/or PSMA- PC3-Flu cell lines, subcutaneous xenografts and disseminated metastatic models. The nanocarriers are efficiently radiolabeled with 177Lu with molar activities 10.8-15.8 MBq/nmol. Besides excellent in vitro PSMA binding affinity (kD = 51.7 nM), the targeted nanocarrier, [177Lu]PEG-(DOTA)1(ACUPA)3, demonstrated excellent in vivo SPECT imaging contrast with 21.3% ID/g PC3-Pip tumors uptake at 192 h. Single doses of 18.5 MBq [177Lu]PEG-(DOTA)1(ACUPA)3 showed complete resolution of the PC3-Pip xenografts observed up to 138 days. Along with PSMA-targeted excellent imaging contrast, these results demonstrated high treatment efficacy of [177Lu]PEG-(DOTA)1(ACUPA)3 for prostate cancer, with potential for clinical translation.
Keywords: enhanced permeability and retention; polymer nanocarriers; prostate cancer; prostate‐specific membrane antigen (PSMA); radioligand therapy; single photon excited computed tomography (SPECT) imaging.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
D. Santi and G. Ashley, are employees of Prolynx Inc. The remaining authors declare no competing financial interest.
Competing interests
The authors declare no competing interests.
Figures





References
-
- Wang H, He Z, Liu X, Huang Y, Hou J, Zhang W, Ding D, Small Structures 2022, 3, 2200036.
-
- Journal of Nuclear Medicine 2022, 63, 13N.
-
- Price E, Orvig C, Chemical Society Reviews 2014, 43, 260. - PubMed
- Hofmann M, Maecke H, Borner A, Weckesser E, Schoffski P, Oei M, Schumacher J, Henze M, Heppeler A, Meyer G, Knapp W, European Journal of Nuclear Medicine 2001, 28, 1751. - PubMed
- Debela D, Muzazu S, Heraro K, Ndalama M, Mesele B, Haile D, Kitui S, Manyazewal T, Sage Open Medicine 2021, 9. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

