Exosomal YB-1 facilitates ovarian restoration by MALAT1/miR-211-5p/FOXO3 axis
- PMID: 38700571
- PMCID: PMC11068691
- DOI: 10.1007/s10565-024-09871-8
Exosomal YB-1 facilitates ovarian restoration by MALAT1/miR-211-5p/FOXO3 axis
Abstract
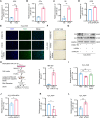
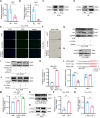
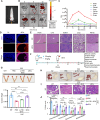
Premature ovarian failure (POF) affects many adult women less than 40 years of age and leads to infertility. Mesenchymal stem cells-derived small extracellular vesicles (MSCs-sEVs) are attractive candidates for ovarian function restoration and folliculogenesis for POF due to their safety and efficacy, however, the key mediator in MSCs-sEVs that modulates this response and underlying mechanisms remains elusive. Herein, we reported that YB-1 protein was markedly downregulated in vitro and in vivo models of POF induced with H2O2 and CTX respectively, accompanied by granulosa cells (GCs) senescence phenotype. Notably, BMSCs-sEVs transplantation upregulated YB-1, attenuated oxidative damage-induced cellular senescence in GCs, and significantly improved the ovarian function of POF rats, but that was reversed by YB-1 depletion. Moreover, YB-1 showed an obvious decline in serum and GCs in POF patients. Mechanistically, YB-1 as an RNA-binding protein (RBP) physically interacted with a long non-coding RNA, MALAT1, and increased its stability, further, MALAT1 acted as a competing endogenous RNA (ceRNA) to elevate FOXO3 levels by sequestering miR-211-5p to prevent its degradation, leading to repair of ovarian function. In summary, we demonstrated that BMSCs-sEVs improve ovarian function by releasing YB-1, which mediates MALAT1/miR-211-5p/FOXO3 axis regulation, providing a possible therapeutic target for patients with POF.
Keywords: MALAT1; POF; YB-1; sEVs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
M6A demethylase FTO-stabilized exosomal circBRCA1 alleviates oxidative stress-induced granulosa cell damage via the miR-642a-5p/FOXO1 axis.J Nanobiotechnology. 2024 Jun 25;22(1):367. doi: 10.1186/s12951-024-02583-5. J Nanobiotechnology. 2024. PMID: 38918838 Free PMC article.
-
miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis.Stem Cell Res Ther. 2019 Nov 29;10(1):360. doi: 10.1186/s13287-019-1442-3. Stem Cell Res Ther. 2019. PMID: 31783913 Free PMC article.
-
Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN.Lab Invest. 2020 Mar;100(3):342-352. doi: 10.1038/s41374-019-0321-y. Epub 2019 Sep 19. Lab Invest. 2020. PMID: 31537899
-
Mesenchymal stem cells therapy: A promising method for the treatment of uterine scars and premature ovarian failure.Tissue Cell. 2022 Feb;74:101676. doi: 10.1016/j.tice.2021.101676. Epub 2021 Nov 9. Tissue Cell. 2022. PMID: 34798583 Review.
-
Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell.J Mol Med (Berl). 2021 May;99(5):637-650. doi: 10.1007/s00109-021-02055-5. Epub 2021 Feb 27. J Mol Med (Berl). 2021. PMID: 33641066 Review.
Cited by
-
Aberrant downregulation of Y-box binding protein 1 expression impairs the cell cycle in an m5C-dependent manner in human granulosa cells from patients with primary ovarian insufficiency.Cell Mol Life Sci. 2025 May 21;82(1):206. doi: 10.1007/s00018-025-05709-6. Cell Mol Life Sci. 2025. PMID: 40397139 Free PMC article.
-
Long Noncoding RNA MALAT1: Salt-Sensitive Hypertension.Int J Mol Sci. 2024 May 18;25(10):5507. doi: 10.3390/ijms25105507. Int J Mol Sci. 2024. PMID: 38791545 Free PMC article. Review.
-
Kuntai Capsules Improve Premature Ovarian Failure by Regulating AMPK-Mediated Autophagy.Reprod Sci. 2025 Aug 11. doi: 10.1007/s43032-025-01949-w. Online ahead of print. Reprod Sci. 2025. PMID: 40789984
References
-
- Akogullari D, Uluer ET, Vatansever HS. Investigation of the relation between follicular atresia and granulosa cells in terms of cell death mechanisms in premature ovarian failure model. Proc. 2018;2(25):1529 (Multidisciplinary Digital Publishing Institute).
-
- Budkina KS, Zlobin NE, Kononova SV, Ovchinnikov LP, Babakov AV. Cold shock domain proteins: Structure and interaction with nucleic acids. Biochemistry (Mosc). 2020;85(Suppl 1):S1-19. - PubMed
-
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18(4):775–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

