ELECT: prospective, randomized trial comparing microvascular plug versus platinum-fibered microcoils for embolization of aneurysm sac side branches before endovascular aortic aneurysm repair
- PMID: 38700601
- PMCID: PMC11068722
- DOI: 10.1186/s42155-024-00454-6
ELECT: prospective, randomized trial comparing microvascular plug versus platinum-fibered microcoils for embolization of aneurysm sac side branches before endovascular aortic aneurysm repair
Abstract
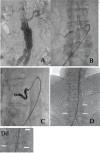
Background: Preemptive selective embolization of aneurysm sac side branches (ASSBs) has been proposed to prevent type II endoleak after endovascular aortic aneurysm repair (EVAR). This study aimed to explore if an embolization strategy using microvascular plugs (MVP) reduces intervention time and radiation dose compared to platinum-fibered microcoils. Furthermore, the effectiveness of the devices in occluding the treated artery was assessed.
Methods: Sixty patients scheduled for EVAR underwent percutaneous preemptive embolization of ASSBs using MVPs or coils after a 1:1 randomization. Follow-up imaging was performed during aortic stentgraft implantation.
Results: Overall, 170 ASSBs were successfully occluded (83 arteries by MVPs and 87 by coils) and no acute treatment failure occurred. The mean procedure time was significantly lower in the group treated with MVPs (55 ± 4 min) compared to coil occlusion (67 ± 3 min; p = 0.018), which was paralleled by a numerically lower radiation dose (119 Gy/cm2 vs. 140 Gy/cm2; p = 0.45). No difference was found for contrast agent use (34 ml MVP group vs 35 ml coil group; p = 0.87). At follow-up, reopening of lumbar arteries was seen in nine cases (four after coil embolization; five after MVPs).
Conclusion: Both microvascular plugs and coils can be effectively used for preemptive embolization of aneurysm sac side branches before EVAR. Use of plugs offers a benefit in terms of intervention time.
Trial registration: ClinicalTrials.gov Identifier: NCT03842930 Registered 15 February 2019.
Keywords: Aortic aneurysm; Coil; EVAR; Embolization; Endoleak; Lumbar artery; Plug.
© 2024. The Author(s).
Conflict of interest statement
M. Konert Consultant for Inari, Speaking Fees: Bayer, Abbott.
A. Schmidt Consultant for Abbott, Boston Scientific, Cook Medical, Cordis, CR Bard, ReFlow Medical, Upstream Peripheral Technologies.
D. Branzan Grants and Speaking Fees from: Artivion, Bentley Innomed GmbH, Cook Medical, Endologix, Getinge, Medtronic.
T. Wittig none.
D. Scheinert Consultant or advisory board member for Abbott, Biotronik, Boston Scientific, Cook Medical, Cordis, CR Bard, Gardia Medical, Medtronic/Covidien, TriReme Medical, Trivascular and Upstream Peripheral Technologies.
S. Steiner Consultant or advisory board member for Boston Scientific, Cook Medical, iThera Medical.
Figures
References
-
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, Investigators Et Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388(10058):2366–74. doi: 10.1016/S0140-6736(16)31135-7. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
