Patient and Health Care Professional Perceptions of the Experience and Impact of Symptoms of Moderate-to-Severe Crohn's Disease in US and Europe: Results from the Cross-Sectional CONFIDE Study
- PMID: 38700629
- PMCID: PMC11258049
- DOI: 10.1007/s10620-024-08434-5
Patient and Health Care Professional Perceptions of the Experience and Impact of Symptoms of Moderate-to-Severe Crohn's Disease in US and Europe: Results from the Cross-Sectional CONFIDE Study
Abstract
Background: Crohn's disease (CD) significantly affects patients' health-related quality of life and well-being.
Aims: Communicating Needs and Features of IBD Experiences (CONFIDE) survey explores the experience and impact of moderate-to-severe CD symptoms on patients' lives and identifies communication gaps between patients and health care professionals (HCPs).
Methods: Online, quantitative, cross-sectional surveys of patients, and HCPs were conducted in the United States (US), Europe (France, Germany, Italy, Spain, United Kingdom), and Japan. Criteria based on previous treatment, steroid use, and/or hospitalization defined moderate-to-severe CD. US and Europe data are presented as descriptive statistics.
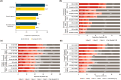
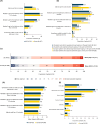
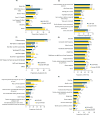
Results: Surveys were completed by 215 US and 547 European patients and 200 US and 503 European HCPs. In both patient groups, top three symptoms currently (past month) experienced were diarrhea, bowel urgency, and increased stool frequency, with more than one-third patients wearing diaper/pad/protection at least once a week in past 3 months due to fear of bowel urgency-related accidents. HCPs ranked diarrhea, blood in stool, and increased stool frequency as the most common symptoms. Although 34.0% US and 27.2% European HCPs ranked bowel urgency among the top five symptoms affecting patient lives, only 12.0% US and 10.9% European HCPs ranked it among top three most impactful symptoms on treatment decisions.
Conclusion: Bowel urgency is common and impactful among patients with CD in the US and Europe. Differences in patient and HCP perceptions of experiences and impacts of bowel urgency exist, with HCPs underestimating its burden. Proactive communication between HCPs and patients in clinical settings is crucial for improving health outcomes in patients with CD.
Keywords: Bowel urgency; Crohn’s disease; Health-related quality of life; Patient experience.
© 2024. The Author(s).
Conflict of interest statement
DTR, Personal fees: Abbvie, Pfizer, Lilly, Janssen, Bristol-Myers Squibb; Grants and personal fees: Takeda; and Board of Trustees, Crohn's & Colitis Foundation (no compensation), CFO, Cornerstones Health (non-profit organization). TH, Personal fees: Abbvie GK, EA Pharma, Janssen Pharmaceutical K.K, JIMRO, Mitsubishi-Tanabe, Mochida Pharmaceutical, Pfizer Japan, Sandoz K.K, Takeda Pharmaceutical, Zeria Pharmaceutical, Celltrion Healthcare Japan, Eli Lilly Japan, Gilead Sciences, Miyarisan Pharmaceutical, Alfresa Pharma Corporation, Kyorin Pharmaceutical; Grants: JIMRO, Mochida Pharmaceutical, Zeria Pharmaceutical, Miyarisan Pharmaceutical, Alfresa Pharma Corporation, and Kyorin Pharmaceutical. SSc, Consultancy fees: AbbVie, Arena, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Falk, Fresenius, Gilead Sciences, IMAB, Janssen, Eli Lilly and Company, Hikma, MSD, Mylan, Pfizer Inc, Protagonist, Provention Bio, Takeda, Ventyx, and Theravance. MCD, Consultancy fees: AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Celgene Corporation, Eli Lilly and Company, F. Hoffman-La Roche Ltd, Genentech Inc., Gilead Sciences, Janssen, LLC, Pfizer, Prometheus Biosciences, Takeda Pharmaceuticals USA, Inc., and UCB; Stock options: Trellus Health Inc.; Research support: AbbVie, Janssen, LLC, Pfizer, and Prometheus Biosciences; and licensing fees: Takeda. RP, Consultancy/speaker fees and/or advisory board member: AbbVie, Amgen, Arena Pharmaceuticals, Alimentiv, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Ferring, Fresenius Kabi, Genentech, Glaxo-Smith Kline, JAMP Bio, Gilead Sciences, Janssen, Merck, Mylan, Novartis, Oppilan Pharma, Organon, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, Sandoz, Shire, Sublimity Therapeutics, and Takeda, Ventyx; Consultancy fees: Abbivax, Abbott, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Galapagos, JAMP Bio, Pendopharm, Satisfai Health, Theravance Biopharma, Trellus, Viatris, and UCB. ST, Grants: AbbVie, ECCO, Eli Lilly and Company, Helmsley Trust International, International Organization for the Study of Inflammatory Bowel Disease, Norman Collision Foundation, Pfizer, Takeda, UCB Pharma, UKIERI, Vifor Pharma; Honoraria: Ferring Pharmaceuticals, Takeda, Eli Lilly and Company, Bristol Myers Squibb, Janssen, Violicom; Travel grants: AbbVie, Eli Lilly and Company, Janssen, Pfizer, Takeda, Ferring Pharmaceuticals, Amgen, Biogen; Consultancy fees: AbbVie, Allergan, Amgen, Apexian, Bioclinica, Biogen, Bristol Myers Squibb, ChemoCentryx, Cosmo Pharmaceuticals, Endpoint Health, Eli Lilly and Company, Enterome, Equillium, Ferring Pharmaceuticals, Genentech/Roche, GlaxoSmithKline, Immunocore, Immunometabolism, Janssen, Merck, Merck Sharp & Dohme, Mestag Therapeutics, Novartis, Pfizer, Protagonist Therapeutics, Proximagen, Receptos, Roche, Sanofi, Satisfai Health, Sensyne Health, Sorriso Pharmaceuticals, Takeda, UCB Pharma, VHsquared, Vifor Pharma. CA, Employment: Adelphi Real World. APB, CK, THG, ADF, SSa, and TP, Employment and stockholder: Eli Lilly and Company.
Figures
Similar articles
-
Broad Impact of Bowel Urgency in Ulcerative Colitis and Crohn's Disease: US, European, and Japanese Patient and Healthcare Professional Perspectives from the Communicating Needs and Features of IBD Experiences (CONFIDE) Survey.Adv Ther. 2025 Sep;42(9):4510-4526. doi: 10.1007/s12325-025-03296-3. Epub 2025 Jul 11. Adv Ther. 2025. PMID: 40643842 Free PMC article.
-
Impact of moderate-to-severe ulcerative colitis and Crohn's disease on sexual activity: United States and European patient perspectives from the communicating needs and features of IBD experiences (CONFIDE) survey.Curr Med Res Opin. 2025 Jun;41(6):1017-1030. doi: 10.1080/03007995.2025.2530736. Epub 2025 Jul 17. Curr Med Res Opin. 2025. PMID: 40635574
-
The Communicating Needs and Features of IBD Experiences (CONFIDE) Study: US and European Patient and Health Care Professional Perceptions of the Experience and Impact of Symptoms of Moderate-to-Severe Ulcerative Colitis.Inflamm Bowel Dis. 2024 Jun 3;30(6):939-949. doi: 10.1093/ibd/izad142. Inflamm Bowel Dis. 2024. PMID: 37603837 Free PMC article.
-
Visceral adiposity and inflammatory bowel disease.Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review.
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
Cited by
-
US and European Patient and Health Care Professional Perspectives on Fatigue in Ulcerative Colitis and Crohn's Disease: Results From the Communicating Needs and Features of Inflammatory Bowel Disease Experiences Survey.Crohns Colitis 360. 2025 Mar 18;7(2):otaf011. doi: 10.1093/crocol/otaf011. eCollection 2025 Apr. Crohns Colitis 360. 2025. PMID: 40364800 Free PMC article.
-
Broad Impact of Bowel Urgency in Ulcerative Colitis and Crohn's Disease: US, European, and Japanese Patient and Healthcare Professional Perspectives from the Communicating Needs and Features of IBD Experiences (CONFIDE) Survey.Adv Ther. 2025 Sep;42(9):4510-4526. doi: 10.1007/s12325-025-03296-3. Epub 2025 Jul 11. Adv Ther. 2025. PMID: 40643842 Free PMC article.
-
Patient and Health Care Professional Perspectives on the Burden and Daily Life Impact of Ulcerative Colitis and Crohn's Disease: Results from the Japanese CONFIDE Study.Adv Ther. 2025 Apr;42(4):1834-1859. doi: 10.1007/s12325-024-03078-3. Epub 2025 Feb 28. Adv Ther. 2025. PMID: 40016440 Free PMC article.
-
The Urgency Numeric Rating Scale: Psychometric Evaluation in Adults with Crohn's Disease.Adv Ther. 2025 Feb;42(2):1044-1060. doi: 10.1007/s12325-024-03081-8. Epub 2024 Dec 18. Adv Ther. 2025. PMID: 39692838 Free PMC article. Clinical Trial.
-
Novel outcomes in inflammatory bowel disease.J Crohns Colitis. 2025 Apr 4;19(4):jjaf040. doi: 10.1093/ecco-jcc/jjaf040. J Crohns Colitis. 2025. PMID: 40078047 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

