Cost-Effectiveness of 20-Valent Pneumococcal Conjugate Vaccine in Argentinean Adults
- PMID: 38700655
- PMCID: PMC11128425
- DOI: 10.1007/s40121-024-00972-9
Cost-Effectiveness of 20-Valent Pneumococcal Conjugate Vaccine in Argentinean Adults
Abstract
Introduction: In Argentina, vaccination with 13-valent pneumococcal conjugate vaccine (PCV13) followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23; PCV13 → PPSV23) has been recommended for all adults aged ≥ 65 years and younger adults with chronic medical ("moderate-risk") or immunocompromising ("high-risk") conditions since 2017. With the approval of a 20-valent PCV (PCV20), we evaluated the cost-effectiveness of PCV20 versus current recommendations for moderate-/high-risk adults aged 18-64 years and all adults 65-99 years.
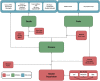
Methods: A probabilistic cohort model was used to project lifetime outcomes and costs associated with invasive pneumococcal disease (IPD) and all-cause non-bacteremic pneumonia (NBP), and the expected impact of vaccination. Clinical outcomes were projected annually based on Argentinean data. Economic costs were estimated based on cases and corresponding medical costs (adjusted to 2023 USD) and costs of vaccine and administration. Cost-effectiveness of PCV20 was evaluated versus the current strategy, PCV13 → PPSV23, and alternatively, versus sequentially administered 15-valent PCV and PPSV23 (PCV15 → PPSV23), and presented as cost per quality-adjusted life year gained; a healthcare system perspective was used. Costs and benefits were discounted at 3%/year.
Results: PCV20 in lieu of PCV13 → PPSV23 among moderate-/high-risk adults aged 18-64 years and all adults 65-99 years (N = 13.4M) prevented 3838 IPD, 4377 inpatient NBP, and 6003 outpatient NBP cases, and 1865 disease-related deaths; relative to PCV15 → PPSV23 the corresponding reductions were 2775, 3285, 4518, and 1348. PCV20 was projected to be the dominant strategy versus PCV13 → PPSV23 and PCV15 → PPSV23 as overall costs were lower by $87.6M and $80.8M, respectively. In probabilistic sensitivity analyses, PCV20 was dominant (i.e., more effective, less costly) in 100% of 1000 simulations.
Conclusions: Analyses suggest implementing a PCV20 vaccination program in moderate-/high-risk adults aged 18-64 years and all adults ≥ 65 years-in lieu of PCV13 → PPSV23-would yield substantial reductions in pneumococcal disease and would be cost saving to the Argentinean healthcare system.
Keywords: Streptococcus pneumoniae; Argentina; Cost-effectiveness; Pneumococcal conjugate vaccine; Vaccination.
Plain language summary
Pneumococcal pneumonia has a high disease burden in both children and adults. Older adults and those with certain underlying conditions are more susceptible to severe pneumococcal disease resulting in considerable economic burden on the healthcare system. In Argentina, vaccination with 13-valent pneumococcal conjugate vaccine (PCV13) followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23) a year later is recommended for all adults aged ≥ 65 years and adults aged 18–64 years with underlying risk conditions. Despite vaccination efforts, prevalence of pneumococcal disease remains high. Two higher-valent PCVs—15-valent PCV (PCV15) and 20-valent PCV (PCV20)—are available for use in adults with PCV20 offering additional serotype coverage. This study assessed the cost-effectiveness of replacing current (PCV13 → PPSV23) and alternative (PCV15 → PPSV23) vaccination strategies with PCV20 alone. The use of PCV20 was evaluated among Argentinean adults aged 18–64 years with underlying risk conditions and all adults aged 65–99 years (N = 13 million). Over a lifetime time horizon, compared to PCV13 → PPSV23, PCV20 use would avert 14,218 cases and 1865 deaths, and increase quality-adjusted life years by 8655. Compared to PCV15 → PPSV23, PCV20 reduced cases and deaths by 10,578 and 1348, respectively, and increased quality-adjusted life years by 6341. In both comparisons, PCV20 use was cost saving with $87.6 million and $80.8 million lower costs compared to PCV13 → PPSV23 and PCV15 → PPSV23, respectively. Results of the cost-effectiveness analyses suggest that the use of PCV20 is a cost-saving strategy, reducing overall costs to the healthcare system and improving public health.
© 2024. Pfizer and Avalere Health.
Conflict of interest statement
Lucila Rey-Ares, Mercedes Mac Mullen, Carolina Carballo and Liping Huang are employed by Pfizer Inc. Ahuva Averin, Dhwani Hariharan, and Mark Atwood are employees of Avalere Health, which received financial support from Pfizer Inc. for this study (including manuscript preparation).
Figures
Similar articles
-
Cost-effectiveness of 15-valent or 20-valent pneumococcal conjugate vaccine for U.S. adults aged 65 years and older and adults 19 years and older with underlying conditions.Vaccine. 2025 Jan 12;44:126567. doi: 10.1016/j.vaccine.2024.126567. Epub 2024 Dec 6. Vaccine. 2025. PMID: 39645434
-
Cost-effectiveness of PCV20 to Prevent Pneumococcal Disease in the Pediatric Population: A German Societal Perspective Analysis.Infect Dis Ther. 2024 Jun;13(6):1333-1358. doi: 10.1007/s40121-024-00977-4. Epub 2024 May 11. Infect Dis Ther. 2024. PMID: 38733494 Free PMC article.
-
Health and economic impact of the 21-valent pneumococcal conjugate vaccine (V116) for adults in Japan: a delta price approach.J Med Econ. 2025 Dec;28(1):136-145. doi: 10.1080/13696998.2024.2445429. Epub 2025 Jan 2. J Med Econ. 2025. PMID: 39705657
-
Systematic literature review of cost-effectiveness analyses of adult 15- and 20-valent pneumococcal vaccines.Vaccine. 2025 Feb 6;46:126656. doi: 10.1016/j.vaccine.2024.126656. Epub 2024 Dec 27. Vaccine. 2025. PMID: 39731806
-
Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults.Pathogens. 2023 May 19;12(5):732. doi: 10.3390/pathogens12050732. Pathogens. 2023. PMID: 37242402 Free PMC article. Review.
Cited by
-
Delayed Transition to 20-Valent Pneumococcal Conjugate Vaccine in Pediatric National Immunization Programs: Forgone Public Health and Economic Benefit.Infect Dis Ther. 2025 Mar;14(3):501-525. doi: 10.1007/s40121-025-01108-3. Epub 2025 Feb 3. Infect Dis Ther. 2025. PMID: 39899200 Free PMC article.
References
-
- Giglio N, et al. 1311. Population-based mortality rates of clinical syndromes potentially associated with pneumococcal disease in Argentina from 2008–2018. Open Forum Infect Dis. 2021;8(Supplement_1):S744.
-
- Minesterio de Salud Argentina, Introducción de la Vacuna Conjugada Contra el Neumococo al Calendario Nacional de Inmunizaciones de la República Argentina; 2011.
-
- Ministerio de Salud Argentina, Vacunación Contra Neumococo. Estrategia Argentina 2017–2018. Lineamientos técnicos manual del vacunador; 2017.
-
- Bonten MJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125. - PubMed
LinkOut - more resources
Full Text Sources

