Timing of Red Blood Cell Transfusions and Occurrence of Necrotizing Enterocolitis: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 38700862
- PMCID: PMC11069076
- DOI: 10.1001/jamanetworkopen.2024.9643
Timing of Red Blood Cell Transfusions and Occurrence of Necrotizing Enterocolitis: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Observational studies often report that anemia and red blood cell (RBC) transfusions are associated with a higher risk of necrotizing enterocolitis (NEC) among extremely low-birthweight (ELBW) infants.
Objective: To evaluate whether there is a temporal association between 72-hour hazard periods of exposure to RBC transfusions and NEC among ELBW infants randomized to either higher or lower hemoglobin transfusion thresholds.
Design, setting, and participants: This post hoc secondary analysis of 1690 ELBW infants who survived to postnatal day 10 enrolled in the Transfusion of Prematures (TOP) randomized multicenter trial between December 1, 2012, and April 12, 2017, was performed between June 2021 and July 2023.
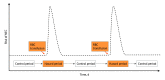
Exposures: First, the distribution of RBC transfusions and the occurrence of NEC up to postnatal day 60 were examined. Second, 72-hour posttransfusion periods were categorized as hazard periods and the pretransfusion periods of variable duration as control periods. Then, the risk of NEC in posttransfusion hazard periods was compared with that in pretransfusion control periods, stratifying the risk based on randomization group (higher or lower hemoglobin transfusion threshold group).
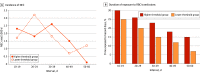
Main outcomes and measures: The primary outcome was incidence of NEC stage 2 or 3. Secondary outcomes included the incidence rates of NEC within five 10-day intervals, taking into account the number of days at risk.
Results: Of 1824 ELBW infants randomized during the TOP trial, 1690 were included in the present analysis (mean [SD] gestational age, 26.0 [1.5] weeks; 899 infants [53.2%] were female). After categorizing 4947 hazard periods and 5813 control periods, we identified 133 NEC cases. Fifty-nine of these cases (44.4%) occurred during hazard periods. Baseline and clinical characteristics of infants with NEC during hazard periods did not differ from those of infants with NEC during control periods. The risk of NEC was 11.9 per 1000 posttransfusion hazard periods and 12.7 per 1000 control periods (adjusted risk ratio, 0.95; 95% CI, 0.68-1.32; P = .74). This risk did not differ significantly between randomization groups, but the incidence rate of NEC per 1000 days peaked between postnatal days 20 and 29 in the lower hemoglobin transfusion threshold group.
Conclusions and relevance: The findings of this post hoc analysis suggest that, among ELBW infants with the hemoglobin ranges occurring in the TOP trial, exposure to RBC transfusions was not temporally associated with a higher risk of NEC during 72-hour posttransfusion hazard periods. Given that the incidence rate of NEC peaked between postnatal days 20 and 29 among infants with lower hemoglobin values, a more in-depth examination of this at-risk period using larger data sets is warranted.
Trial registration: ClinicalTrials.gov Identifier: NCT01702805.
Conflict of interest statement
Figures
References
-
- Strauss RG. Practical issues in neonatal transfusion practice. Am J Clin Pathol. 1997;107(4)(suppl 1):S57-S63. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- UG1 HD068284/HD/NICHD NIH HHS/United States
- UG1 HD040492/HD/NICHD NIH HHS/United States
- UG1 HD021364/HD/NICHD NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- U01 HD036790/HD/NICHD NIH HHS/United States
- U01 HL112748/HL/NHLBI NIH HHS/United States
- UG1 HD034216/HD/NICHD NIH HHS/United States
- U01 HL112776/HL/NHLBI NIH HHS/United States
- U10 HD021373/HD/NICHD NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UG1 HD027851/HD/NICHD NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UG1 HD027904/HD/NICHD NIH HHS/United States
- UG1 HD068270/HD/NICHD NIH HHS/United States
- U10 HD027880/HD/NICHD NIH HHS/United States
- UG1 HD021385/HD/NICHD NIH HHS/United States
- UL1 TR000442/TR/NCATS NIH HHS/United States
- U24 HD095254/HD/NICHD NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- UG1 HD040689/HD/NICHD NIH HHS/United States
- UG1 HD053109/HD/NICHD NIH HHS/United States
- K23 HD102554/HD/NICHD NIH HHS/United States
- UG1 HD068244/HD/NICHD NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UG1 HD087226/HD/NICHD NIH HHS/United States
- UG1 HD068278/HD/NICHD NIH HHS/United States
- UG1 HD053089/HD/NICHD NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UG1 HD068263/HD/NICHD NIH HHS/United States
- UG1 HD027856/HD/NICHD NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

