Techniques for subretinal injections in animals
- PMID: 38700998
- PMCID: PMC11911964
- DOI: 10.1111/vop.13219
Techniques for subretinal injections in animals
Abstract
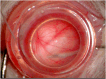
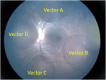
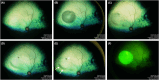
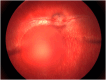
Subretinal injections are not commonly performed during clinical treatment of animals but are frequently used in laboratory animal models to assess therapeutic efficacy and safety of gene and cell therapy products. Veterinary ophthalmologists are often employed to perform the injections in the laboratory animal setting, due to knowledge of comparative ocular anatomy between species and familiarity with operating on non-human eyes. Understanding the different approaches used for subretinal injection in each species and potential complications that may be encountered is vital to achieving successful and reproducible results. This manuscript provides a summary of different approaches to subretinal injections in the most common animal model species, along with information from published literature and experience of the authors to educate novice or experienced surgeons tasked with performing these injections for the first time.
Keywords: animal model; retina; subretinal injection.
© 2024 The Authors. Veterinary Ophthalmology published by Wiley Periodicals LLC on behalf of American College of Veterinary Ophthalmologists.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Optimized technique for subretinal injections in mice.Methods Mol Biol. 2013;935:343-9. doi: 10.1007/978-1-62703-080-9_24. Methods Mol Biol. 2013. PMID: 23150380
-
An Alternative and Validated Injection Method for Accessing the Subretinal Space via a Transcleral Posterior Approach.J Vis Exp. 2016 Dec 7;(118):54808. doi: 10.3791/54808. J Vis Exp. 2016. PMID: 28060316 Free PMC article.
-
Effect of subretinal injection on retinal structure and function in a rat oxygen-induced retinopathy model.Mol Vis. 2017 Nov 29;23:832-843. eCollection 2017. Mol Vis. 2017. PMID: 29259390 Free PMC article.
-
How Advanced are Nanocarriers for Effective Subretinal Injection?Int J Nanomedicine. 2024 Sep 10;19:9273-9289. doi: 10.2147/IJN.S479327. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39282576 Free PMC article. Review.
-
Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases.Ophthalmic Res. 2017;58(4):217-226. doi: 10.1159/000479157. Epub 2017 Sep 1. Ophthalmic Res. 2017. PMID: 28858866 Review.
Cited by
-
Advances in technical methods and applications of subretinal injections in experimental animals.Front Vet Sci. 2025 Apr 30;12:1574519. doi: 10.3389/fvets.2025.1574519. eCollection 2025. Front Vet Sci. 2025. PMID: 40370828 Free PMC article. Review.
References
-
- Haupert CL, McCuen BW, Jaffe GJ, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid–gas exchange for displacement of thick submacular hemorrhage in age‐related macular degeneration. Am J Ophthalmol. 2001;131:208‐215. - PubMed
-
- Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231‐2239. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

