Patient and Public Involvement and Engagement in the Development of a Platform Clinical Trial for Parkinson's Disease: An Evaluation Protocol
- PMID: 38701161
- PMCID: PMC11191543
- DOI: 10.3233/JPD-230444
Patient and Public Involvement and Engagement in the Development of a Platform Clinical Trial for Parkinson's Disease: An Evaluation Protocol
Abstract
Background: Patient and public involvement and engagement (PPIE) in the design of trials is important, as participant experience critically impacts delivery. The Edmond J Safra Accelerating Clinical Trials in PD (EJS ACT-PD) initiative is a UK consortium designing a platform trial for disease modifying therapies in PD.
Objective: The integration of PPIE in all aspects of trial design and its evaluation throughout the project.
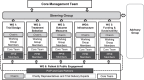
Methods: PwP and care partners were recruited to a PPIE working group (WG) via UK Parkinson's charities, investigator patient groups and participants of a Delphi study on trial design. They are supported by charity representatives, trial delivery experts, researchers and core project team members. PPIE is fully embedded within the consortium's five other WGs and steering group. The group's terms of reference, processes for effective working and PPIE evaluation were co-developed with PPIE contributors.
Results: 11 PwP and 4 care partners have supported the PPIE WG and contributed to the development of processes for effective working. A mixed methods research-in-action study is ongoing to evaluate PPIE within the consortium. This includes the Patient Engagement in Research Scale -a quantitative PPIE quality measure; semi-structured interviews -identifying areas for improvement and overall impressions of involvement; process fidelity- recording adherence; project documentation review - identifying impact of PPIE on project outputs.
Conclusions: We provide a practical example of PPIE in complex projects. Evaluating feasibility, experiences and impact of PPIE involvement in EJS ACT-PD will inform similar programs on effective strategies. This will help enable future patient-centered research.
Keywords: Evaluation study; Parkinson’s disease; clinical trial protocol; patient and public involvement.
Conflict of interest statement
CBC, TF, ELL and KMF are Editorial Board members of this journal, but were not involved in the peer-review process nor had access to any information regarding its peer review.
Figures

References
-
- Aiyegbusi OL, McMullan C, Hughes SE, Turner GM, Subramanian A, Hotham R, Davies EH, Frost C, Alder Y, Agyen L, Buckland L, Camaradou J, Chong A, Jeyes F, Kumar S, Matthews KL, Moore P, Ormerod J, Price G, Saint-Cricq M, Stanton D, Walker A, Haroon S, Denniston AK, Calvert MJ, TLC Study Group (2023) Considerations for patient and public involvement and engagement in health research. Nat Med 29, 1922–1929. - PubMed
-
- Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, Knox C, Nadin B, Rothwell J, Surtees M, Julious SA (2017) Recruitment and retention of participants in randomised controlled trials: A review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 7, e015276. - PMC - PubMed
-
- Rochester L, Carroll C (2022) Implications of research that excludes under-served populations. Nat Rev Neurol 18, 449–450. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

