Perspectives on improving photosynthesis to increase crop yield
- PMID: 38701340
- PMCID: PMC11449117
- DOI: 10.1093/plcell/koae132
Perspectives on improving photosynthesis to increase crop yield
Abstract
Improving photosynthesis, the fundamental process by which plants convert light energy into chemical energy, is a key area of research with great potential for enhancing sustainable agricultural productivity and addressing global food security challenges. This perspective delves into the latest advancements and approaches aimed at optimizing photosynthetic efficiency. Our discussion encompasses the entire process, beginning with light harvesting and its regulation and progressing through the bottleneck of electron transfer. We then delve into the carbon reactions of photosynthesis, focusing on strategies targeting the enzymes of the Calvin-Benson-Bassham (CBB) cycle. Additionally, we explore methods to increase carbon dioxide (CO2) concentration near the Rubisco, the enzyme responsible for the first step of CBB cycle, drawing inspiration from various photosynthetic organisms, and conclude this section by examining ways to enhance CO2 delivery into leaves. Moving beyond individual processes, we discuss two approaches to identifying key targets for photosynthesis improvement: systems modeling and the study of natural variation. Finally, we revisit some of the strategies mentioned above to provide a holistic view of the improvements, analyzing their impact on nitrogen use efficiency and on canopy photosynthesis.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. K.N. is an inventor on a patent “Transgenic plants with increased photosynthesis efficiency and growth” US20230183731A1, and K.N. and D.P.-T. are inventors on a patent application “Methods of screening for plant gain of function mutations and compositions therefor” US20230323480A1. All other authors declare no competing interests.
Figures





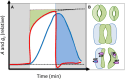

References
Publication types
MeSH terms
Substances
Grants and funding
- 862201/European Union's Horizon2020 Research and Innovation Programme
- Dutch Organization for Scientific Research
- 101082091/European Horizon Research and Innovation Actions
- CE140100015/Australian Research Council Centre of Excellence for Translational Photosynthesis
- Realising Improved Photosynthetic Efficiency
- University of Essex
- University of Illinois, USA
- GATES/Bill & Melinda Gates Foundation/United States
- DFID
- FFAR
- BB/J004138/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 862201/European Union's Horizon2020 Research and Innovation Programme
- 862201/European Union's Horizon2020 Research and Innovation Programme
- Realizing Increased Photosynthetic Efficiency
- 57248/Bill & Melinda Gates Agricultural Innovations
- Lancaster University
- BB/X011003/1/BBSRC Institute Strategic Programme: Delivering Sustainable Wheat
- AP23-1_023/Carbon Technology Research Foundation
- Foreign, Commonwealth and Development Office
- University of Edinburgh
- Princeton University, USA
- US Department of Energy
- Office of Science
- Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division
- Realizing Increased Photosynthetic Efficiency
- OPP1172157)/U.K. Foreign, Commonwealth & Development Office
- Berkeley Fellowship
- DGE 1752814/NSF Graduate Research Fellowship Program
- HHMI/Howard Hughes Medical Institute/United States
- European Union's H2020 research and innovation
- Horizon programs
- 862087/GAIN4CROPS
- 101082091/BEST-CROP
- Deutsche Forschungsgemeinschaft
- Cluster of Excellence for Plant Sciences

