Can Incorporating Molecular Testing Improve the Accuracy of Newborn Screening for Congenital Adrenal Hyperplasia?
- PMID: 38701341
- PMCID: PMC11531606
- DOI: 10.1210/clinem/dgae297
Can Incorporating Molecular Testing Improve the Accuracy of Newborn Screening for Congenital Adrenal Hyperplasia?
Abstract
Context: Single-tier newborn screening (NBS) for congenital adrenal hyperplasia (CAH) using 17-hydroxyprogesterone (17OHP) measured by fluoroimmunoassay (FIA) in samples collected at 24 to 48 hours produces a high false-positive rate (FPR). Second-tier steroid testing can reduce the FPR and has been widely implemented.
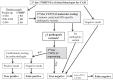
Objective: We investigated the accuracy of an alternative multitier CAH NBS protocol that incorporates molecular testing of the CYP21A2 gene and reduces the first-tier 17OHP cutoff to minimize missed cases.
Methods: We create a Minnesota-specific CYP21A2 pathogenic variants panel; developed a rapid, high-throughput multiplex, allele-specific-primer-extension assay; and performed a 1-year retrospective analysis of Minnesota NBS results comparing metrics between a conventional steroid-based 2-tier protocol and a molecular-based multitier NBS protocol, applied post hoc.
Results: CYP21A2 gene sequencing of 103 Minnesota families resulted in a Minnesota-specific panel of 21 pathogenic variants. The Centers for Disease Control and Prevention created a molecular assay with 100% accuracy and reproducibility. Two-tier steroid-based screening of 68 659 live births during 2015 resulted in 2 false negatives (FNs), 91 FPs, and 1 true positive (TP). A 3-tier protocol with a lower first-tier steroid cutoff, second-tier 21-variant CYP21A2 panel, and third-tier CYP21A2 sequencing would have resulted in 0 FNs, 52 FPs, and 3 TPs.
Conclusion: Incorporation of molecular testing could improve the accuracy of CAH NBS, although some distinct challenges of molecular testing may need to be considered before implementation by NBS programs.
Keywords: CYP21A2 gene; congenital adrenal hyperplasia; newborn screening; premature infants.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Sarafoglou K, Banks K, Gaviglio A, Hietala A, McCann M, Thomas W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics. 2012;130(5):e1261‐e1268. - PubMed
-
- Sarafoglou K, Banks K, Kyllo J, Pittock S, Thomas W. Cases of congenital adrenal hyperplasia missed by newborn screening in Minnesota. JAMA. 2012;307(22):2371‐2374. - PubMed
-
- Sarafoglou K, Gaviglio A, Hietala A, et al. . Comparison of newborn screening protocols for congenital adrenal hyperplasia in preterm infants. J Pediatr. 2014;164(5):1136‐1140. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

