Metabolic Reprogramming of Tumor-Associated Macrophages Using Glutamine Antagonist JHU083 Drives Tumor Immunity in Myeloid-Rich Prostate and Bladder Cancers
- PMID: 38701369
- PMCID: PMC11217738
- DOI: 10.1158/2326-6066.CIR-23-1105
Metabolic Reprogramming of Tumor-Associated Macrophages Using Glutamine Antagonist JHU083 Drives Tumor Immunity in Myeloid-Rich Prostate and Bladder Cancers
Abstract
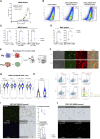
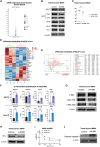
Glutamine metabolism in tumor microenvironments critically regulates antitumor immunity. Using the glutamine-antagonist prodrug JHU083, we report potent tumor growth inhibition in urologic tumors by JHU083-reprogrammed tumor-associated macrophages (TAMs) and tumor-infiltrating monocytes. We show JHU083-mediated glutamine antagonism in tumor microenvironments induced by TNF, proinflammatory, and mTORC1 signaling in intratumoral TAM clusters. JHU083-reprogrammed TAMs also exhibited increased tumor cell phagocytosis and diminished proangiogenic capacities. In vivo inhibition of TAM glutamine consumption resulted in increased glycolysis, a broken tricarboxylic acid (TCA) cycle, and purine metabolism disruption. Although the antitumor effect of glutamine antagonism on tumor-infiltrating T cells was moderate, JHU083 promoted a stem cell-like phenotype in CD8+ T cells and decreased the abundance of regulatory T cells. Finally, JHU083 caused a global shutdown in glutamine-utilizing metabolic pathways in tumor cells, leading to reduced HIF-1α, c-MYC phosphorylation, and induction of tumor cell apoptosis, all key antitumor features. Altogether, our findings demonstrate that targeting glutamine with JHU083 led to suppressed tumor growth as well as reprogramming of immunosuppressive TAMs within prostate and bladder tumors that promoted antitumor immune responses. JHU083 can offer an effective therapeutic benefit for tumor types that are enriched in immunosuppressive TAMs.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S. Yegnasubramanian reports grants from the NIH during the conduct of the study, as well as grants and personal fees from Cepheid, other support from Digital Harmonic and Brahm Astra Therapeutics, and grants from Bristol Meyers Squibb and Janssen outside the submitted work. R.D. Leone reports grants from the NIH during the conduct of the study; personal fees from Mitobridge and Abilita outside the submitted work; and a patent for methods for cancer and immunotherapy using prodrugs of glutamine analogs. Patent # 10842763 was issued, licensed, and with royalties paid from Dracen Pharmaceuticals, Inc. R. Rais reports patents for US10954257B2 and US20230009398A1 issued and licensed to Dracen Pharmaceuticals, Inc. R. Rais is an inventor on multiple Johns Hopkins University (JHU) patents covering novel glutamine antagonist prodrugs. These patents have been licensed to Dracen Pharmaceuticals, Inc. R. Rais is also a cofounder of and holds equity in Dracen Pharmaceuticals, Inc., and served as a scientific consultant to Dracen Pharmaceuticals, Inc. This arrangement has been reviewed and approved by the JHU in accordance with its conflict-of-interest policies. R. Rais declares no other conflict. B.S. Slusher reports grants and personal fees from Dracen Pharmaceuticals, Inc. outside the submitted work and patents for US 16/754053, US 16/262476, and US 16/454880 issued and licensed to Dracen Pharmaceuticals, Inc. B.S. Slusher is an inventor on multiple JHU patents covering novel glutamine antagonist prodrugs and their utility. These patents have been licensed to Dracen Pharmaceuticals, Inc. B.S. Slusher is also a cofounder of and holds equity in Dracen Pharmaceuticals, Inc. and also served as a scientific consultant to Dracen Pharmaceuticals, Inc. This arrangement has been reviewed and approved by the JHU in accordance with its conflict-of-interest policies. D.M. Pardoll reports other support from Dracen Pharmaceuticals, Inc. during the conduct of the study and a patent licensed to Dracen Pharmaceuticals, Inc. J.D. Powell reports other support from Dracen Pharmaceuticals, Inc. during the conduct of the study; other support from Calico outside the submitted work; and a patent for DRP104 issued and licensed. J.C. Zarif reports grants from the NIH/NCI, the Prostate Cancer Foundation Young Investigator Award, the Maryland Cigarette Restitution Fund, and the Bloomberg∼Kimmel Institute for Cancer Immunotherapy during the conduct of the study. No disclosures were reported by the other authors.
Figures




References
-
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55. - PubMed
-
- Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab 2019;30:36–50. - PubMed
-
- Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 2018;17:887–904. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

