ABBV-319: a CD19-targeting glucocorticoid receptor modulator antibody-drug conjugate therapy for B-cell malignancies
- PMID: 38701407
- PMCID: PMC11375461
- DOI: 10.1182/blood.2024023849
ABBV-319: a CD19-targeting glucocorticoid receptor modulator antibody-drug conjugate therapy for B-cell malignancies
Abstract
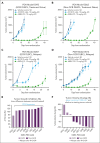
Glucocorticoids are key components of the standard-of-care treatment regimens for B-cell malignancy. However, systemic glucocorticoid treatment is associated with several adverse events. ABBV-319 is a CD19-targeting antibody-drug conjugate engineered to reduce glucocorticoid-associated toxicities while possessing 3 distinct mechanisms of action (MOA) to increase therapeutic efficacy: (1) antibody-mediated delivery of a glucocorticoid receptor modulator (GRM) payload to activate apoptosis, (2) inhibition of CD19 signaling, and (3) enhanced fragment crystallizable (Fc)-mediated effector function via afucosylation of the antibody backbone. ABBV-319 elicited potent GRM-driven antitumor activity against multiple malignant B-cell lines in vitro, as well as in cell line-derived xenografts and patient-derived xenografts (PDXs) in vivo. Remarkably, a single dose of ABBV-319 induced sustained tumor regression and enhanced antitumor activity compared with repeated dosing of systemic prednisolone at the maximum tolerated dose in mice. The unconjugated CD19 monoclonal antibody (mAb) also displayed antiproliferative activity in a subset of B-cell lymphoma cell lines through the inhibition of phosphoinositide 3-kinase signaling. Moreover, afucosylation of CD19 mAb enhanced Fc-mediated antibody-dependent cellular cytotoxicity. Notably, ABBV-319 displayed superior efficacy compared with afucosylated CD19 mAb in human CD34+ peripheral blood mononuclear cell-engrafted NSG-Tg(Hu-IL15) transgenic mice, demonstrating enhanced antitumor activity when multiple MOAs are enabled. ABBV-319 also showed durable antitumor activity across multiple B-cell lymphoma PDX models, including nongerminal center B-cell diffuse large B-cell lymphoma and relapsed lymphoma after R-CHOP treatment. Collectively, these data support the ongoing evaluation of ABBV-319 in a phase 1 clinical trial.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.A.C., E.E., A.L.D., W.Z., C.C., K.P., D.M., E.P., A.O., P.E., L.R., R.D., C.H., L.L., W.A., P.Z., W.L., A.H.J., K.M., Z.Z., G.R., Z.C., Y.L., J.C., G.Z., T.C., M.B., C.C.M., A.H., M. McPherson, T.U., M.A.P., X.Z., J.H., M. McDevitt, K.J.F., S.M.-L., and J.W.P. are employees of AbbVie. O.P. and A.B. were employees of AbbVie at the time of the study. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of the data, review, and approval of the manuscript.
Figures







Comment in
-
'Roids without rage via B-cell-targeted ADC?Blood. 2024 Aug 15;144(7):686-687. doi: 10.1182/blood.2024025123. Blood. 2024. PMID: 39145946 No abstract available.
References
-
- Livingston RB, Carter SK. 1st ed. Springer; 1970. Single Agents in Cancer Chemotherapy.
-
- Pearson OH, Eliel LP. Use of pituitary adrenocorticotropic hormone (ACTH) and cortisone in lymphomas and leukemias. J Am Med Assoc. 1950;144(16):1349–1353. - PubMed
-
- Kofman S, Perlia CP, Boesen E, Eisenstein R, Taylor SG., 3rd The role of corticosteroids in the treatment of malignant lymphomas. Cancer. 1962;15:338–345. - PubMed
-
- Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328(14):1002–1006. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

