Dietary intake and glutamine-serine metabolism control pathologic vascular stiffness
- PMID: 38701775
- PMCID: PMC11152997
- DOI: 10.1016/j.cmet.2024.04.010
Dietary intake and glutamine-serine metabolism control pathologic vascular stiffness
Abstract
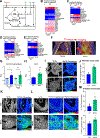
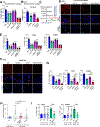
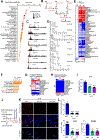
Perivascular collagen deposition by activated fibroblasts promotes vascular stiffening and drives cardiovascular diseases such as pulmonary hypertension (PH). Whether and how vascular fibroblasts rewire their metabolism to sustain collagen biosynthesis remains unknown. Here, we found that inflammation, hypoxia, and mechanical stress converge on activating the transcriptional coactivators YAP and TAZ (WWTR1) in pulmonary arterial adventitial fibroblasts (PAAFs). Consequently, YAP and TAZ drive glutamine and serine catabolism to sustain proline and glycine anabolism and promote collagen biosynthesis. Pharmacologic or dietary intervention on proline and glycine anabolic demand decreases vascular stiffening and improves cardiovascular function in PH rodent models. By identifying the limiting metabolic pathways for vascular collagen biosynthesis, our findings provide guidance for incorporating metabolic and dietary interventions for treating cardiopulmonary vascular disease.
Keywords: cardiovascular disease; collagen metabolism; fibrosis; glutamine metabolism; metabolism; nutrition; pulmonary hypertension; serine metabolism; vascular fibroblast.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.Y.C. has served as a consultant for Merck, Janssen, and United Therapeutics. S.Y.C. is a director, officer, and shareholder in Synhale Therapeutics. S.Y.C. has held research grants from WoodNext, Bayer, and United Therapeutics. S.Y.C. and T.B. have filed patent applications regarding the targeting of metabolism in pulmonary hypertension. G.Y., Z.-K.Y., and O.O. are listed as inventors in patents not related to this work, which are filed by MSKCC. O.O. receives royalties from MSKCC, Johnson & Johnson, Jazz, and Y-mAbs and owns shares in Angiogenex, for which he is an unpaid member of the SAB, all of which are not related to this work.
Figures




References
-
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. (2020). Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol 76, 2982–3021. 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang Y-Y, et al. (2015). Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep 13, 1016–1032. 10.1016/j.celrep.2015.09.049. - DOI - PMC - PubMed
-
- Kothapalli D, Liu S-L, Bae YH, Monslow J, Xu T, Hawthorne EA, Byfield FJ, Castagnino P, Rao S, Rader DJ, et al. (2012). Cardiovascular protection by apoE and apoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Rep 2, 1259–1271. 10.1016/j.celrep.2012.09.018. - DOI - PMC - PubMed
-
- Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, et al. (2017). Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 136, 2468–2485. 10.1161/CIRCULATIONAHA.117.028069. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

