Microglial CMPK2 promotes neuroinflammation and brain injury after ischemic stroke
- PMID: 38701781
- PMCID: PMC11148565
- DOI: 10.1016/j.xcrm.2024.101522
Microglial CMPK2 promotes neuroinflammation and brain injury after ischemic stroke
Abstract
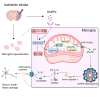
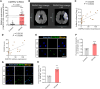
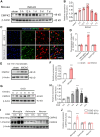
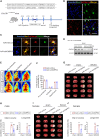
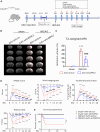
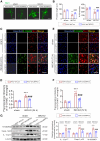
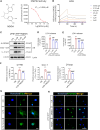
Neuroinflammation plays a significant role in ischemic injury, which can be promoted by oxidized mitochondrial DNA (Ox-mtDNA). Cytidine/uridine monophosphate kinase 2 (CMPK2) regulates mtDNA replication, but its role in neuroinflammation and ischemic injury remains unknown. Here, we report that CMPK2 expression is upregulated in monocytes/macrophages and microglia post-stroke in humans and mice, respectively. Microglia/macrophage CMPK2 knockdown using the Cre recombination-dependent adeno-associated virus suppresses the inflammatory responses in the brain, reduces infarcts, and improves neurological outcomes in ischemic CX3CR1Cre/ERT2 mice. Mechanistically, CMPK2 knockdown limits newly synthesized mtDNA and Ox-mtDNA formation and subsequently blocks NLRP3 inflammasome activation in microglia/macrophages. Nordihydroguaiaretic acid (NDGA), as a CMPK2 inhibitor, is discovered to reduce neuroinflammation and ischemic injury in mice and prevent the inflammatory responses in primary human monocytes from ischemic patients. Thus, these findings identify CMPK2 as a promising therapeutic target for ischemic stroke and other brain disorders associated with neuroinflammation.
Keywords: CMPK2; NLRP3 inflammasome; Ox-mtDNA; human PBMC cells; microglia; stroke.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

